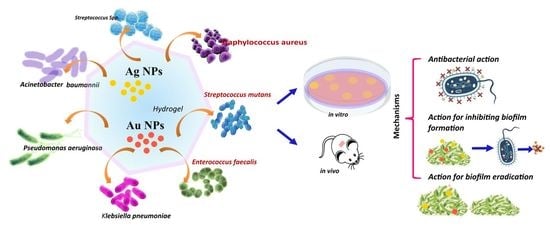
Advanced Hydrogels Combined with Silver and Gold Nanoparticles against Antimicrobial Resistance
Abstract
:1. Introduction
| NPs | Bacteria | Mechanism of Action | Ref. |
|---|---|---|---|
| Ag | E. coli, B. subtilis, and S. aureus |
| [20,46] |
| Au | P. aeruginosa and E. coli |
| [47,48,49,50] |
| ZnO | E. coli, S. aureus, and Botrytis cinerea |
| [51,52,53] |
| TiO2 | E. coli and Bacillus megaterium |
| [54,55] |
| Cu | E. coli and Bacillus subtilis |
| [56] |
| MgO | E. coli, S. aureus, Bacillus subtilis, and Bacillus megaterium |
| [57,58] |
2. Silver Nanoparticles (Ag NPs)
2.1. Antibacterial Activity of Ag NPs Loaded into Hydrogels
2.2. Antibiofilm Activity of Hydrogels Loaded with Ag NPs
3. Gold Nanoparticles (Au NPs)
3.1. Antibacterial of Au NPs Loaded into Hydrogels
3.2. Antibiofilm Activity of Au NPs Loaded into Hydrogels
4. Mechanism of Action of Ag NPs and Au NPs Loaded into Hydrogels
4.1. Mechanism of Antibacterial Action
4.2. Mechanism of Action for Inhibiting Biofilm Formation
4.3. Mechanism for Biofilm Eradication
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tommasi, R.; Brown, D.G.; Walkup, G.K.; Manchester, J.I.; Miller, A.A. ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 2015, 14, 529–542. [Google Scholar] [CrossRef]
- Shrivastava, S.; Shrivastava, P.; Ramasamy, J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018, 32, 76. [Google Scholar] [CrossRef]
- Piddock, L.J.V. The crisis of no new antibiotics—What is the way forward? Lancet Infect. Dis. 2012, 12, 249–253. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002, 1, 895–910. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013; Centres for Disease Control and Prevention, US Department of Health and Human Services: Altanta, GA, USA, 2013. [Google Scholar]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [Green Version]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial biofilms. J. Cell Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshehri, S.M.; Aldalbahi, A.; Al-Hajji, A.B.; Chaudhary, A.A.; Panhuis, M.I.H.; Alhokbany, N.; Ahamad, T. Development of carboxymethyl cellulose-based hydrogel and nanosilver composite as antimicrobial agents for UTI pathogens. Carbohydr. Polym. 2016, 138, 229–236. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, E.M.P.; Martins, C.C.B.; de Oliveira Santos, J.V.; da Silva, W.R.C.; Silva, S.B.C.; Pelagio-Flores, M.A.; Galembeck, A.; Cavalcanti, I.M.F. Silver nanoparticles–chitosan composites activity against resistant bacteria: Tolerance and biofilm inhibition. J. Nanopart. Res. 2021, 23, 196. [Google Scholar] [CrossRef]
- Andrei, S.; Droc, G.; Stefan, G. FDA approved antibacterial drugs: 2018–2019. Discoveries 2019, 7, e102. [Google Scholar] [CrossRef]
- Baym, M.; Lieberman, T.D.; Kelsic, E.D.; Chait, R.; Gross, R.; Yelin, I.; Kishony, R. Spatiotemporal microbial evolution on antibiotic landscapes. Science 2016, 353, 1147–1151. [Google Scholar] [CrossRef] [Green Version]
- Gerência de Vigilância e Monitoramento em Serviços de Saúde; Gerência Geral de Tecnologia em Serviços de Saúde; Agência Nacional de Vigilância Sanitária. ANVISA: Boletim de segurança do paciente e qualidade em serviços de saúde nº 14: Avaliação dos indicadores nacionais das infecções relacionadas à assistência à saúde (IRAS) e resistência microbiana do ano de 2015; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2016; Volume 14, pp. 1–116. [Google Scholar]
- Gerência de Vigilância e Monitoramento em Serviços de Saúde; Gerência Geral de Tecnologia em Serviços de Saúde; Agência Nacional de Vigilância Sanitária. Plano Nacional para a Prevenção e o Controle da Resistência Microbiana nos Serviços de Saúde; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2017; Volume 84. [Google Scholar]
- Perelshtein, I.; Lipovsky, A.; Perkas, N.; Gedanken, A.; Moschini, E.; Mantecca, P. The influence of the crystalline nature of nano-metal oxides on their antibacterial and toxicity properties. Nano Res. 2015, 8, 695–707. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
- Lakshminarayanan, R.; Ye, E.; Young, D.J.; Li, Z.; Loh, X.J. Recent Advances in the Development of Antimicrobial Nanoparticles for Combating Resistant Pathogens. Adv. Healthc. Mater. 2018, 7, 1701400. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.G.; Huang, J.J.; Wu, X.W.; Ren, Y.H.; Li, Z.A.; Ren, J.A. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef]
- Soliman, W.E.; Elsewedy, H.S.; Younis, N.S.; Shinu, P.; Elsawy, L.E.; Ramadan, H.A. Evaluating Antimicrobial Activity and Wound Healing Effect of Rod-Shaped Nanoparticles. Polymers 2022, 14, 2637. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Mansourpanah, Y. Construction of chitosan-carboxymethyl beta-cyclodextrin silver nanocomposite hydrogel to improve antibacterial activity. Plast. Rubber Compos. 2018, 47, 273–281. [Google Scholar] [CrossRef]
- Kojic, N.; Pritchard, E.M.; Tao, H.; Brenckle, M.A.; Mondia, J.P.; Panilaitis, B.; Omenetto, F.; Kaplan, D.L. Focal Infection Treatment using Laser-Mediated Heating of Injectable Silk Hydrogels with Gold Nanoparticles. Adv. Funct. Mater. 2012, 22, 3793–3798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.S.; Zhang, H.M.; Yang, X.; Zhang, W.H.; Jiang, M.; Wen, T.; Wang, J.; Guo, R.; Liu, H.J. Preparation and Application of Quaternized Chitosan- and AgNPs-Base Synergistic Antibacterial Hydrogel for Burn Wound Healing. Molecules 2021, 26, 4037. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Ferraz, M.P.; Monteiro, F.J.; Fernandes, M.H.; Beppu, M.M.; Mantione, D.; Sardon, H. Antibacterial silk fibroin/nanohydroxyapatite hydrogels with silver and gold nanoparticles for bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Majhi, R.K.; Singh, A.; Mishra, M.; Tiwari, A.; Chawla, S.; Guha, P.; Satpati, B.; Mohapatra, H.; Goswami, L.; et al. Carbohydrate-Coated Gold–Silver Nanoparticles for Efficient Elimination of Multidrug Resistant Bacteria and in Vivo Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 42998–43017. [Google Scholar] [CrossRef]
- Edhari, B.A.; Mashreghi, M.; Makhdoumi, A.; Darroudi, M. Antibacterial and antibiofilm efficacy of Ag NPs, Ni NPs and Al2O3 NPs singly and in combination against multidrug-resistant Klebsiella pneumoniae isolates. J. Trace Elem. Med. Biol. 2021, 68, 126840. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; AlYahya, S.; Jalal, M.; Khan, H.M.; Asiri, S.M.M.; Ahmad, W.; Mahdi, A.A.; et al. Biogenic Gold Nanoparticles as Potent Antibacterial and Antibiofilm Nano-Antibiotics against Pseudomonas aeruginosa. Antibiotics 2020, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, C.; Zhou, L.; Hu, Q.; Kong, Y.; Song, D.; Cheng, Y.; Zhang, Y. A smart hydrogel for on-demand delivery of antibiotics and efficient eradication of biofilms. Sci. China Mater. 2021, 64, 1035–1046. [Google Scholar] [CrossRef]
- Rezaei, N.; Hamidabadi, H.G.; Khosravimelal, S.; Zahiri, M.; Ahovan, Z.A.; Bojnordi, M.N.; Eftekhari, B.S.; Hashemi, A.; Ganji, F.; Darabi, S.; et al. Antimicrobial peptides-loaded smart chitosan hydrogel: Release behavior and antibacterial potential against antibiotic resistant clinical isolates. Int. J. Biol. Macromol. 2020, 164, 855–862. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Antibiofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential antibiofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcelik, B.; Ho, K.K.K.; Glattauer, V.; Willcox, M.; Kumar, N.; Thissen, H. Poly(ethylene glycol)-Based Coatings Combining Low-Biofouling and Quorum-Sensing Inhibiting Properties to Reduce Bacterial Colonization. ACS Biomater. Sci. Eng. 2017, 3, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandian, M.; Selvaprithviraj, V.; Pradeep, A.; Rangasamy, J. In-situ silver nanoparticles incorporated N, O-carboxymethyl chitosan based adhesive, self-healing, conductive, antibacterial and antibiofilm hydrogel. Int. J. Biol. Macromol. 2021, 188, 501–511. [Google Scholar] [CrossRef]
- Carpa, R.; Remizovschi, A.; Culda, C.A.; Butiuc-Keul, A.L. Inherent and Composite Hydrogels as Promising Materials to Limit Antimicrobial Resistance. Gels 2022, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Matai, I.; Sachdev, A. Toward Designing of Anti-infective Hydrogels for Orthopedic Implants: From Lab to Clinic. ACS Biomater. Sci. Eng. 2021, 7, 1933–1961. [Google Scholar] [CrossRef]
- Ferrag, C.; Li, S.P.; Jeon, K.; Andoy, N.M.; Sullan, R.M.A.; Mikhaylichenko, S.; Kerman, K. Polyacrylamide hydrogels doped with different shapes of silver nanoparticles: Antibacterial and mechanical properties. Colloids Surf. B-Biointerfaces 2021, 197, 111397. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Kwiatkowska, A.; Drabik, M.; Lipko, A.; Grzeczkowicz, A.; Stachowiak, R.; Marszalik, A.; Granicka, L.H. Composite Membrane Dressings System with Metallic Nanoparticles as an Antibacterial Factor in Wound Healing. Membranes 2022, 12, 215. [Google Scholar] [CrossRef]
- Haidari, S.; FFA, I.J.; Metsemakers, W.J.; Maarse, W.; Vogely, H.C.; Ramsden, A.J.; McNally, M.A.; Govaert, G.A.M. The Role of Negative-Pressure Wound Therapy in Patients with Fracture-Related Infection: A Systematic Review and Critical Appraisal. BioMed Res. Int. 2021, 2021, 7742227. [Google Scholar] [CrossRef]
- Haidari, H.; Bright, R.; Garg, S.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Eradication of Mature Bacterial Biofilms with Concurrent Improvement in Chronic Wound Healing Using Silver Nanoparticle Hydrogel Treatment. Biomedicines 2021, 9, 1182. [Google Scholar] [CrossRef]
- Banerjee, D.; Shivapriya, P.M.; Gautam, P.K.; Misra, K.; Sahoo, A.K.; Samanta, S.K. A Review on Basic Biology of Bacterial Biofilm Infections and Their Treatments by Nanotechnology-Based Approaches. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 243–259. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLoS ONE 2014, 9, e108876. [Google Scholar] [CrossRef] [PubMed]
- Bowman, M.C.; Ballard, T.E.; Ackerson, C.J.; Feldheim, D.L.; Margolis, D.M.; Melander, C. Inhibition of HIV fusion with multivalent gold nanoparticles. J. Am. Chem. Soc. 2008, 130, 6896–6897. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small Molecule-Capped Gold Nanoparticles as Potent Antibacterial Agents That Target Gram-Negative Bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf. B Biointerfaces 2010, 80, 45–50. [Google Scholar] [CrossRef]
- Baram-Pinto, D.; Shukla, S.; Gedanken, A.; Sarid, R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small (Weinh. Der Bergstr. Ger.) 2010, 6, 1044–1050. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Min, L.; Zhang, W.; Liu, J.; Hou, Z.; Chu, M.; Li, L.; Shen, W.; Zhao, Y.; Zhang, H. Zinc Oxide Nanoparticles Influence Microflora in Ileal Digesta and Correlate Well with Blood Metabolites. Front. Microbiol. 2017, 8, 992. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Darbari, S.; Abdi, Y.; Haghighi, F.; Mohajerzadeh, S.; Haghighi, N. Investigating the antifungal activity of TiO2nanoparticles deposited on branched carbon nanotube arrays. J. Phys. D Appl. Phys. 2011, 44, 245401. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Manivasakan, P.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Impact of nano and bulk ZrO2, TiO2 particles on soil nutrient contents and PGPR. J. Nanosci. Nanotechnol. 2013, 13, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Tejeda, L.; Malpartida, F.; Esteban-Cubillo, A.; Pecharromán, C.; Moya, J.S. Antibacterial and antifungal activity of a soda-lime glass containing copper nanoparticles. Nanotechnology 2009, 20, 505701. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Lv, B.-F. MgO nanoparticles as antibacterial agent: Preparation and activity. Braz. J. Chem. Eng. 2014, 31, 591–601. [Google Scholar] [CrossRef]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lou, Z.; Zhang, X.; Hu, X.; Zhang, H. A simple strategy to fabricate poly (acrylamide-co-alginate)/gold nanocomposites for inactivation of bacteria. Appl. Phys. A 2014, 117, 2009–2018. [Google Scholar] [CrossRef]
- Lu, B.; Ye, H.; Shang, S.; Xiong, Q.; Yu, K.; Li, Q.; Xiao, Y.; Dai, F.; Lan, G. Novel wound dressing with chitosan gold nanoparticles capped with a small molecule for effective treatment of multiantibiotic-resistant bacterial infections. Nanotechnology 2018, 29, 425603. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical Intermediate-Modified Gold Nanoparticles: Against Multidrug-Resistant Bacteria and Wound-Healing Application via an Electrospun Scaffold. ACS Nano 2017, 11, 5737–5745. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Badhwar, R.; Mangla, B.; Neupane, Y.R.; Khanna, K.; Popli, H. Quercetin loaded silver nanoparticles in hydrogel matrices for diabetic wound healing. Nanotechnology 2021, 32, 505102. [Google Scholar] [CrossRef]
- Varaprasad, K.; Reddy, G.S.M.; Jayaramudu, J.; Sadiku, R.; Ramam, K.; Ray, S.S. Development of microbial resistant Carbopol nanocomposite hydrogels via a green process. Biomater. Sci. 2014, 2, 257–263. [Google Scholar] [CrossRef]
- Ou, Q.; Huang, K.; Fu, C.; Huang, C.; Fang, Y.; Gu, Z.; Wu, J.; Wang, Y. Nanosilver-incorporated halloysite nanotubes/gelatin methacrylate hybrid hydrogel with osteoimmunomodulatory and antibacterial activity for bone regeneration. Chem. Eng. J. 2020, 382, 123019. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, Y.; Zhao, Y.; Wang, D.; Luan, Y. Microwave synthesis of graphene oxide decorated with silver nanoparticles for slow-release antibacterial hydrogel. Mater. Today Commun. 2022, 31, 103663. [Google Scholar] [CrossRef]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of Citrate, PVP, and PEG Coated Silver Nanoparticles in Ecotoxicology Media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Ge, J.; Li, Y.; Wang, M.; Gao, C.; Yang, S.; Lei, B. Engineering conductive antioxidative antibacterial nanocomposite hydrogel scaffolds with oriented channels promotes structure-functional skeletal muscle regeneration. Chem. Eng. J. 2021, 425, 130333. [Google Scholar] [CrossRef]
- Deng, Z.; Li, M.; Hu, Y.; He, Y.; Tao, B.; Yuan, Z.; Wang, R.; Chen, M.; Luo, Z.; Cai, K. Injectable biomimetic hydrogels encapsulating Gold/metal–organic frameworks nanocomposites for enhanced antibacterial and wound healing activity under visible light actuation. Chem. Eng. J. 2021, 420, 129668. [Google Scholar] [CrossRef]
- Nešović, K.; Mišković-Stanković, V. Silver/poly(vinyl alcohol)/graphene hydrogels for wound dressing applications: Understanding the mechanism of silver, antibacterial agent release. J. Vinyl Addit. Technol. 2022, 28, 196–210. [Google Scholar] [CrossRef]
- Nešović, K.; Janković, A.; Perić-Grujić, A.; Vukašinović-Sekulić, M.; Radetić, T.; Živković, L.; Park, S.-J.; Yop Rhee, K.; Mišković-Stanković, V. Kinetic models of swelling and thermal stability of silver/poly(vinyl alcohol)/chitosan/graphene hydrogels. J. Ind. Eng. Chem. 2019, 77, 83–96. [Google Scholar] [CrossRef]
- Nesovic, K.; Jankovic, A.; Kojic, V.; Vukasinovic-Sekulic, M.; Peric-Grujic, A.; Rhee, K.Y.; Miskovic-Stankovic, V. Silver/poly(vinyl alcohol)/chitosan/graphene hydrogels—Synthesis, biological and physicochemical properties and silver release kinetics. Compos. Part B-Eng. 2018, 154, 175–185. [Google Scholar] [CrossRef]
- Kujda, M.; Wieja, M.; Adamczyk, Z.; BocheSka, O.; Bra, G.; Kozik, A.; BielaSka, E.; Barbasz, J. Charge Stabilized Silver Nanoparticles Applied as Antibacterial Agents. J. Nanosci. Nanotechnol. 2015, 15, 3574–3583. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N. Biosynthesis of Ag and Cu NPs by secondary metabolites of usnic acid and thymol with biological macromolecules aggregation and antibacterial activities against multi drug resistant (MDR) bacteria. Int. J. Biol. Macromol. 2019, 128, 893–901. [Google Scholar] [CrossRef]
- Aurore, V.; Caldana, F.; Blanchard, M.; Kharoubi Hess, S.; Lannes, N.; Mantel, P.-Y.; Filgueira, L.; Walch, M. Silver-nanoparticles increase bactericidal activity and radical oxygen responses against bacterial pathogens in human osteoclasts. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 601–607. [Google Scholar] [CrossRef]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Feizi, S.; Taghipour, E.; Ghadam, P.; Mohammadi, P. Antifungal, antibacterial, antibiofilm and colorimetric sensing of toxic metals activities of eco friendly, economical synthesized Ag/AgCl nanoparticles using Malva Sylvestris leaf extracts. Microb. Pathog. 2018, 125, 33–42. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. JBIC J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Yusuf, M. Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 2343–2356. [Google Scholar]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Ahmad, M.K.; Mahdi, A.A.; Pal, R.; Cameotra, S.S. Interaction of silver nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Basic Microbiol. 2014, 54, 905–915. [Google Scholar] [CrossRef]
- Mirzajani, F.; Ghassempour, A.; Aliahmadi, A.; Esmaeili, M.A. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res. Microbiol. 2011, 162, 542–549. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, J.H.; Chae, Y.J.; Kim, Y.S.; Kim, B.C.; Sang, B.-I.; Gu, M.B. Analysis of the Toxic Mode of Action of Silver Nanoparticles Using Stress-Specific Bioluminescent Bacteria. Small 2008, 4, 746–750. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C. Chemiosmotic Mechanism of Antimicrobial Activity of Ag+ in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [Green Version]
- Gnanadhas, D.P.; Ben Thomas, M.; Thomas, R.; Raichur, A.M.; Chakravortty, D. Interaction of Silver Nanoparticles with Serum Proteins Affects Their Antimicrobial Activity In Vivo. Antimicrob. Agents Chemother. 2013, 57, 4945–4955. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, B.; Garmaroudi, F.S.; Hashemi, M.; Nezhad, H.R.; Nasrollahi, A.; Ardalan, S.; Ardalan, S. Comparison of the anti-bacterial activity on the nanosilver shapes: Nanoparticles, nanorods and nanoplates. Adv. Powder Technol. 2012, 23, 22–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, H.; Huang, W.; Zhou, Y.; Yan, D. Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J. Colloid Interface Sci. 2008, 325, 371–376. [Google Scholar] [CrossRef]
- Ivask, A.; ElBadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity Mechanisms in Escherichia coli Vary for Silver Nanoparticles and Differ from Ionic Silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 16, 53. [Google Scholar] [CrossRef] [Green Version]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Banerjee, M.; Mallick, S.; Paul, A.; Chattopadhyay, A.; Ghosh, S.S. Heightened Reactive Oxygen Species Generation in the Antimicrobial Activity of a Three Component Iodinated Chitosan−Silver Nanoparticle Composite. Langmuir 2010, 26, 5901–5908. [Google Scholar] [CrossRef]
- Mishra, S.K.; Raveendran, S.; Ferreira, J.M.F.; Kannan, S. In Situ Impregnation of Silver Nanoclusters in Microporous Chitosan-PEG Membranes as an Antibacterial and Drug Delivery Percutaneous Device. Langmuir 2016, 32, 10305–10316. [Google Scholar] [CrossRef]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, B.; Yao, Q.; Yang, Y.; Xie, J.; Yan, N. Recent advances in the synthesis and catalytic applications of ligand-protected, atomically precise metal nanoclusters. Coord. Chem. Rev. 2016, 322, 1–29. [Google Scholar] [CrossRef]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef]
- Luo, Z.; Zheng, K.; Xie, J. Engineering ultrasmall water-soluble gold and silver nanoclusters for biomedical applications. Chem. Commun. 2014, 50, 5143–5155. [Google Scholar] [CrossRef]
- Díez, I.; Eronen, P.; Österberg, M.; Linder, M.B.; Ikkala, O.; Ras, R.H.A. Functionalization of Nanofibrillated Cellulose with Silver Nanoclusters: Fluorescence and Antibacterial Activity. Macromol. Biosci. 2011, 11, 1185–1191. [Google Scholar] [CrossRef]
- Wang, X.; Gao, W.; Xu, S.; Xu, W. Luminescent fibers: In situ synthesis of silver nanoclusters on silk via ultraviolet light-induced reduction and their antibacterial activity. Chem. Eng. J. 2012, 210, 585–589. [Google Scholar] [CrossRef]
- Balagna, C.; Irfan, M.; Perero, S.; Miola, M.; Maina, G.; Santella, D.; Simone, A. Characterization of antibacterial silver nanocluster/silica composite coating on high performance Kevlar® textile. Surf. Coat. Technol. 2017, 321, 438–447. [Google Scholar] [CrossRef]
- Willing, B.P.; Pepin, D.M.; Marcolla, C.S.; Forgie, A.J.; Diether, N.E.; Bourrie, B.C.T. Bacterial resistance to antibiotic alternatives: A wolf in sheep’s clothing? Anim. Front. 2018, 8, 39–47. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wen, H.; Rao, Z.; Zhu, C.; Liu, M.; Min, L.; Fan, L.; Tao, S. Preparation and characterization of chitosan–collagen peptide/oxidized konjac glucomannan hydrogel. Int. J. Biol. Macromol. 2018, 108, 376–382. [Google Scholar] [CrossRef]
- Qin, D.; Zhang, A.; Wang, N.; Yao, Y.; Chen, X.; Liu, Y. Hydroxybutyl chitosan/ oxidized glucomannan self-healing hydrogels as BMSCs-derived exosomes carriers for advanced stretchable wounds. Appl. Mater. Today 2022, 26, 101342. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, G.; Liu, J.; Li, M.; Li, Q.; Tang, K. Gelatin/Oxidized Konjac Glucomannan Composite Hydrogels with High Resistance to Large Deformation for Tissue Engineering Applications. ACS Appl. Bio Mater. 2021, 4, 1536–1543. [Google Scholar] [CrossRef]
- Wu, H.; Bu, N.; Chen, J.; Chen, Y.; Sun, R.; Wu, C.; Pang, J. Construction of Konjac Glucomannan/Oxidized Hyaluronic Acid Hydrogels for Controlled Drug Release. Polymers 2022, 14, 927. [Google Scholar] [CrossRef]
- Chen, H.; Lan, G.; Ran, L.; Xiao, Y.; Yu, K.; Lu, B.; Dai, F.; Wu, D.; Lu, F. A novel wound dressing based on a Konjac glucomannan/silver nanoparticle composite sponge effectively kills bacteria and accelerates wound healing. Carbohydr. Polym. 2018, 183, 70–80. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Mei, L.; Dubey, N.; Fenno, J.C.; Piva, E.; Lund, R.G.; Schwendeman, A.; Bottino, M.C. Injectable MMP-Responsive Nanotube-Modified Gelatin Hydrogel for Dental Infection Ablation. ACS Appl. Mater. Interfaces 2020, 12, 16006–16017. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Xu, X.-H.N. Synthesis and characterization of tunable rainbow colored colloidal silver nanoparticles using single-nanoparticle plasmonic microscopy and spectroscopy. J. Mater. Chem. 2010, 20, 9867–9876. [Google Scholar] [CrossRef]
- de Lacerda Coriolano, D.; de Souza, J.B.; Bueno, E.V.; Medeiros, S.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Qiu, W.; Li, M.; Li, N.; Li, X.; Qin, X.; Wang, X.; Yu, J.; Li, F.; Huang, L.; et al. Multifunctional hydrogel platform for biofilm scavenging and O2 generating with photothermal effect on diabetic chronic wound healing. J. Colloid Interface Sci. 2022, 617, 542–556. [Google Scholar] [CrossRef]
- Imran, M.; Hussain, S.; Mehmood, K.; Saeed, Z.; Parvaiz, M.; Younas, U.; Nadeem, H.A.; Ghalani, S.P.; Saleem, S. Optimization of ecofriendly synthesis of Ag nanoparticles by Linum usitatissimum hydrogel using response surface methodology and its biological applications. Mater. Today Commun. 2021, 29, 102789. [Google Scholar] [CrossRef]
- Alfuraydi, R.T.; Alminderej, F.M.; Mohamed, N.A. Evaluation of Antimicrobial and Antibiofilm Formation Activities of Novel Poly(vinyl alcohol) Hydrogels Reinforced with Crosslinked Chitosan and Silver Nano-Particles. Polymers 2022, 14, 1619. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.; Alvarado-Gomez, E.; Magaña-Aquino, M.; Sánchez-Sánchez, R.; Velasquillo, C.; Gonzalez, C.; Ganem-Rondero, A.; Martínez-Castañon, G.; Zavala-Alonso, N.; Martinez-Gutierrez, F. Antibiofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 317–323. [Google Scholar] [CrossRef]
- Katas, H.; Mohd Akhmar, M.A.; Suleman Ismail Abdalla, S. Biosynthesized silver nanoparticles loaded in gelatine hydrogel for a natural antibacterial and antibiofilm wound dressing. J. Bioact. Compat. Polym. 2021, 36, 111–123. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, B.; Liu, R.; Dong, Y.; Zhao, Y.; Wu, Y. Development of pH-responsive nanocomposites with remarkably synergistic antibiofilm activities based on ultrasmall silver nanoparticles in combination with aminoglycoside antibiotics. Colloids Surf. B Biointerfaces 2021, 208, 112112. [Google Scholar] [CrossRef]
- Lopez-Carrizales, M.; Mendoza-Mendoza, E.; Peralta-Rodriguez, R.D.; Pérez-Díaz, M.A.; Portales-Pérez, D.; Magaña-Aquino, M.; Aragón-Piña, A.; Infante-Martínez, R.; Barriga-Castro, E.D.; Sánchez-Sánchez, R.; et al. Characterization, antibiofilm and biocompatibility properties of chitosan hydrogels loaded with silver nanoparticles and ampicillin: An alternative protection to central venous catheters. Colloids Surf. B Biointerfaces 2020, 196, 111292. [Google Scholar] [CrossRef]
- Wunnoo, S.; Bilhman, S.; Waen-ngoen, T.; Yawaraya, S.; Paosen, S.; Lethongkam, S.; Kaewnopparat, N.; Voravuthikunchai, S.P. Thermosensitive hydrogel loaded with biosynthesized silver nanoparticles using Eucalyptus camaldulensis leaf extract as an alternative treatment for microbial biofilms and persistent cells in tissue infections. J. Drug Deliv. Sci. Technol. 2022, 74, 103588. [Google Scholar] [CrossRef]
- Kaul, S.; Sagar, P.; Gupta, R.; Garg, P.; Priyadarshi, N.; Singhal, N.K. Mechanobactericidal, Gold Nanostar Hydrogel-Based Bandage for Bacteria-Infected Skin Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 44084–44097. [Google Scholar] [CrossRef] [PubMed]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the Next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Chitra, G.; Franklin, D.S.; Sudarsan, S.; Sakthivel, M.; Guhanathan, S. Noncytotoxic silver and gold nanocomposite hydrogels with enhanced antibacterial and wound healing applications. Polym. Eng. Sci. 2018, 58, 2133–2142. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N.; Hikmat, S.; Abu Ghith, D.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Int. J. Pharm. 2019, 565, 174–186. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Yang, J.; Liu, W. Bacteria activated-macrophage membrane-coated tough nanocomposite hydrogel with targeted photothermal antibacterial ability for infected wound healing. Chem. Eng. J. 2021, 420, 127638. [Google Scholar] [CrossRef]
- Al-Bakri, A.G.; Mahmoud, N.N. Photothermal-Induced Antibacterial Activity of Gold Nanorods Loaded into Polymeric Hydrogel against Pseudomonas aeruginosa Biofilm. Molecules 2019, 24, 2661. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Vecchio, D.; Li, J.; Zhu, J.; Zhang, Q.; Fu, V.; Li, J.; Thamphiwatana, S.; Lu, D.; Zhang, L. Hydrogel Containing Nanoparticle-Stabilized Liposomes for Topical Antimicrobial Delivery. ACS Nano 2014, 8, 2900–2907. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, C.; Alcock, E.; Buttimer, F.; Schmidt, M.; Clarke, D.; Pemble, M.; Bardosova, M. Synthesis and characterisation of cross-linked chitosan composites functionalised with silver and gold nanoparticles for antimicrobial applications. Sci. Technol. Adv. Mater. 2017, 18, 528–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Akbari-Fakhrabadi, A.; Carrasco-Sánchez, V.; Amalraj, J. Hydroxypropyl methylcellulose-copper nanoparticle and its nanocomposite hydrogel films for antibacterial application. Carbohydr. Polym. 2021, 254, 117302. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef]
- Chitra, G.; Selvi, M.S.; Franklin, D.S.; Sudarsan, S.; Sakthivel, M.; Guhanathan, S. pH-sensitive biopolymeric hydrogel-based on indole-3-acetic acid for wound healing and anti-cancer applications. SN Appl. Sci. 2019, 1, 1641. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines 2019, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Hui, P.C.-L.; Kan, C.-W.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef] [Green Version]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.D.; Alston, M.; Stringer, M.F.; Betts, R.P.; Baranyi, J.; et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Guo, J.; Zhang, Q.; Zhu, S.; Liu, L.; Jiang, X.; Wei, D.-H.; Liu, R.-S.; Li, L. Gelatin sponge functionalized with gold/silver clusters for antibacterial application. Nanotechnology 2020, 31, 134004. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, Z.; Liu, Y.; Zhang, W.; Geng, L.; Ni, T. Incorporation of ROS-Responsive Substance P-Loaded Zeolite Imidazolate Framework-8 Nanoparticles into a Ca(2+)-Cross-Linked Alginate/Pectin Hydrogel for Wound Dressing Applications. Int. J. Nanomed. 2020, 15, 333–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prusty, K.; Swain, S.K. Release of ciprofloxacin drugs by nano gold embedded cellulose grafted polyacrylamide hybrid nanocomposite hydrogels. Int. J. Biol. Macromol. 2019, 126, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.-K.; Wang, Y.; Ren, R.; Zheng, W.; Liao, W. Gold Nanoparticle: Recent Progress on Its Antibacterial Applications and Mechanisms. J. Nanomater. 2021, 2021, 2501345. [Google Scholar] [CrossRef]
- Abdalla, S.S.I.; Katas, H.; Azmi, F.; Busra, M.F.M. Antibacterial and Antibiofilm Biosynthesised Silver and Gold Nanoparticles for Medical Applications: Mechanism of Action, Toxicity and Current Status. Curr. Drug Deliv. 2020, 17, 88–100. [Google Scholar] [CrossRef]
- Bermúdez-Jiménez, C.; Niño-Martínez, N.; Patiño-Marín, N.; Martínez-Gutiérrez, F.; Ruiz, F.; Bach, H.; Martínez-Castañón, G. Effective control of biofilms by photothermal therapy using a gold nanorod hydrogel. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 333–342. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Ju, M.; Milbrandt, N.B.; Tsai, Y.H.; Navarreto-Lugo, M.; Visperas, A.; Klika, A.; Barsoum, W.; Higuera-Rueda, C.A.; Samia, A.C.S. Photoactivated Gold Nanorod Hydrogel Composite Containing d-Amino Acids for the Complete Eradication of Bacterial Biofilms on Metal Alloy Implant Materials. ACS Appl. Nano Mater. 2020, 3, 5862–5873. [Google Scholar] [CrossRef]
- Galdámez-Falla, V.-M.; Castillo-Martínez, J.-C.; de Alba-Montero, I.; Patiño-Marín, N.; Niño-Martínez, N.; Ruiz, F.; Martínez-Castañón, G.-A. Formation of a Mature Biofilm of Enterococcus Faecalis in Root Canal and Its Treatment Using Gold Nanorods. J. Mater. Sci. Res. Rev. 2022, 9, 31–43. [Google Scholar]
- Kabiri, F.; Aghaei, S.S.; Pourbabaee, A.A.; Soleimani, M.; Komeili Movahhed, T. Antibiofilm and cytotoxic potential of extracellular biosynthesized gold nanoparticles using actinobacteria Amycolatopsis sp. KMN. Prep. Biochem. Biotechnol. 2022, 1–14. [Google Scholar] [CrossRef]
- Sahoo, B.; Panigrahi, L.L.; Das, R.P.; Pradhan, A.K.; Arakha, M. Biogenic Synthesis of Silver Nanoparticle from Punica granatum L. and Evaluation of Its Antioxidant, Antimicrobial and Antibiofilm Activity. J. Inorg. Organometall. Polym. Mater. 2022, 32, 4250–4259. [Google Scholar] [CrossRef]
- Dharul Salam, F.; Nadar Vinita, M.; Puja, P.; Prakash, S.; Yuvakkumar, R.; Kumar, P. Anti-bacterial and antibiofilm efficacies of bioinspired gold nanoparticles. Mater. Lett. 2020, 261, 126998. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Jers, C.; Joshi, A.S.; Garnæs, J.; Mijakovic, I. Silver nanoparticles produced from Cedecea sp. exhibit antibiofilm activity and remarkable stability. Sci. Rep. 2021, 11, 12619. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, G.; Gohil, J.; Gohil, N.; Chaudhari, H.; Gangapuram, B.; Khambhati, K.; Maurya, R.; Alzahrani, K.J.; Ramakrishna, S.; Singh, V. Biosynthesis and characterization of Serratia marcescens derived silver nanoparticles: Investigating its antibacterial, antibiofilm potency and molecular docking analysis with biofilm-associated proteins. J. Mol. Liq. 2022, 365, 120094. [Google Scholar] [CrossRef]
- Lavaee, F.; Motamedifar, M.; Rafiee, G. The effect of photodynamic therapy by gold nanoparticles on Streptococcus mutans and biofilm formation: An in vitro study. Lasers Med. Sci. 2022, 37, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Selem, E.; Mekky, A.F.; Hassanein, W.A.; Reda, F.M.; Selim, Y.A. Antibacterial and antibiofilm effects of silver nanoparticles against the uropathogen Escherichia coli U12. Saudi J. Biol. Sci. 2022, 29, 103457. [Google Scholar] [CrossRef]
- Rajivgandhi, G.N.; Ramachandran, G.; Maruthupandy, M.; Manoharan, N.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Li, W.-J. Anti-oxidant, anti-bacterial and antibiofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumoniae. Colloids Surf. A 2020, 600, 124830. [Google Scholar] [CrossRef]
- Aksoy, İ.; Küçükkeçeci, H.; Sevgi, F.; Metin, Ö.; Hatay Patir, I. Photothermal Antibacterial and Antibiofilm Activity of Black Phosphorus/Gold Nanocomposites against Pathogenic Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 26822–26831. [Google Scholar] [CrossRef] [PubMed]
- Govindappa, M.; Tejashree, S.; Thanuja, V.; Hemashekhar, B.; Srinivas, C.; Nasif, O.; Pugazhendhi, A.; Raghavendra, V.B. Pomegranate fruit fleshy pericarp mediated silver nanoparticles possessing antimicrobial, antibiofilm formation, antioxidant, biocompatibility and anticancer activity. J. Drug Deliv. Sci. Technol. 2021, 61, 102289. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, L.; Yang, Y.; Yin, Z.; Guo, B. Biodegradable gelatin/silver nanoparticle composite cryogel with excellent antibacterial and antibiofilm activity and hemostasis for Pseudomonas aeruginosa-infected burn wound healing. J. Colloid Interface Sci. 2022, 608, 2278–2289. [Google Scholar] [CrossRef]
- Mostafa, E.M.; Abdelgawad, M.A.; Musa, A.; Alotaibi, N.H.; Elkomy, M.H.; Ghoneim, M.M.; Badawy, M.S.E.M.; Taha, M.N.; Hassan, H.M.; Hamed, A.A. Chitosan Silver and Gold Nanoparticle Formation Using Endophytic Fungi as Powerful Antimicrobial and Antibiofilm Potentialities. Antibiotics 2022, 11, 668. [Google Scholar] [CrossRef]
- Rini, P.; Sugiharto; Agusniar Furkani, L.; Masfufatun; Lusiani, T.; Noer Kumala, I. Antibacterial and antibiofilm effect of silver and gold nanoparticles in Uropathogenic Escherichia coli. Berk. Penelit. Hayati 2021, 27, 67–72. [Google Scholar] [CrossRef]
- Neihaya, H.Z.; Zaman, H.H. Investigating the effect of biosynthesized silver nanoparticles as antibiofilm on bacterial clinical isolates. Microb. Pathog. 2018, 116, 200–208. [Google Scholar] [CrossRef]
- Hetta, H.F.; Al-Kadmy, I.M.S.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Ellah, N.H.A.; Ahmed, E.A.; Abd-ellatief, R.B.; El-Masry, E.A.; et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef]
- Ghar, S.B.; Das, Y.K. The green & eco-friendly biosynthesized biocompatible metallic silver nanoparticles for anti-bacterial and antibiofilm efficacy. Eur. J. Biotechnol. Biosci. 2022, 10, 43–49. [Google Scholar]
- Majumdar, M.; Biswas, S.C.; Choudhury, R.; Upadhyay, P.; Adhikary, A.; Roy, D.N.; Misra, T.K. Synthesis of Gold Nanoparticles Using Citrus macroptera Fruit Extract: Antibiofilm and Anticancer Activity. ChemistrySelect 2019, 4, 5714–5723. [Google Scholar] [CrossRef]
- Khan, F.; Lee, J.W.; Manivasagan, P.; Pham, D.T.N.; Oh, J.; Kim, Y.M. Synthesis and characterization of chitosan oligosaccharide-capped gold nanoparticles as an effective antibiofilm drug against the Pseudomonas aeruginosa PAO1. Microb. Pathog. 2019, 135, 103623. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Khadri, H.; Azam, M.; Khan, M.A.; Rahmani, A.H.; Alrumaihi, F.; Khateef, R.; Ansari, M.A.; Alatawi, E.A.; Alsugoor, M.H.; et al. Ajwa-Dates (Phoenix dactylifera)-Mediated Synthesis of Silver Nanoparticles and Their Anti-Bacterial, Antibiofilm, and Cytotoxic Potential. Appl. Sci. 2022, 12, 4537. [Google Scholar] [CrossRef]
- Hussain, A.; Alajmi, M.F.; Khan, M.A.; Pervez, S.A.; Ahmed, F.; Amir, S.; Husain, F.M.; Khan, M.S.; Shaik, G.M.; Hassan, I.; et al. Biosynthesized Silver Nanoparticle (AgNP) From Pandanus odorifer Leaf Extract Exhibits Anti-metastasis and Antibiofilm Potentials. Front. Microbiol. 2019, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Qais, F.A.; Ahmad, I.; Altaf, M.; Manoharadas, S.; Al-Rayes, B.F.; Ali Abuhasil, M.S.; Almaroai, Y.A. Biofabricated silver nanoparticles exhibit broad-spectrum antibiofilm and antiquorum sensing activity against Gram-negative bacteria. RSC Adv. 2021, 11, 13700–13710. [Google Scholar] [CrossRef]
- Khan, F.; Park, S.K.; Bamunuarachchi, N.I.; Oh, D.; Kim, Y.M. Caffeine-loaded gold nanoparticles: Antibiofilm and anti-persister activities against pathogenic bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 3717–3731. [Google Scholar] [CrossRef]
- Qais, F.A.; Ahmad, I.; Altaf, M.; Alotaibi, S.H. Biofabrication of Gold Nanoparticles Using Capsicum annuum Extract and Its Antiquorum Sensing and Antibiofilm Activity against Bacterial Pathogens. ACS Omega 2021, 6, 16670–16682. [Google Scholar] [CrossRef]
- El-Telbany, M.; El-Sharaki, A. Antibacterial and antibiofilm activity of silver nanoparticles on multi-drug resistance pseudomonas aeruginosa isolated from dental-implant. J. Oral Biol. Craniofac. Res. 2022, 12, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle-Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.B.; Raffaelli, S.; Mitchell, S.G.; Faccio, R.; Alborés, S. Biofilm Eradication Using Biogenic Silver Nanoparticles. Molecules 2020, 25, 2023. [Google Scholar] [CrossRef] [PubMed]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal Oxide Nanoparticles Against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef]
- Sokary, R.; Abu el-naga, M.N.; Bekhit, M.; Atta, S. A potential antibiofilm, antimicrobial and anticancer activities of chitosan capped gold nanoparticles prepared by γ–irradiation. Mater. Technol. 2022, 37, 493–502. [Google Scholar] [CrossRef]
- Wong, C.W.; Chan, Y.S.; Jeevanandam, J.; Pal, K.; Bechelany, M.; Abd Elkodous, M.; El-Sayyad, G.S. Response Surface Methodology Optimization of Mono-dispersed MgO Nanoparticles Fabricated by Ultrasonic-Assisted Sol–Gel Method for Outstanding Antimicrobial and Antibiofilm Activities. J. Clust. Sci. 2020, 31, 367–389. [Google Scholar] [CrossRef]
- Hussein, N.; Khadum, M.M. Evaluation of the Biosynthesized Silver Nanoparticles” Effects on Biofilm Formation. J. Appl. Sci. Nanotechnol. 2021, 1, 23–31. [Google Scholar] [CrossRef]
- Dridi, R.; Essghaier, B.; Hannachi, H.; Khedher, G.B.; Chaffei, C.; Zid, M.F. Biosynthesized silver nanoparticles using Anagallis monelli: Evaluation of antioxidant activity, antibacterial and antifungal effects. J. Mol. Struct. 2022, 1251, 132076. [Google Scholar] [CrossRef]




| System | Materials | Ag NP Properties (Size and Surface Charge) | NP Synthesis Method | Bacteria | Target | Antibacterial Properties: Inhibition Zone (mm) and MIC Values | Ref. |
|---|---|---|---|---|---|---|---|
| Ag–ODex HA-ADH/HACC | Dextran, sodium hyaluronic, chitosan quaternary ammonium salt, and AgNO3 | 50–190 nm | Chemical reduction, in situ, Schiff-base reaction to form hydrogel | E. coli ATCC8739, S. aureus ATCC14458, and P. aeruginosa CMCCB10104 | In vitro; In vivo, rats | The Kirby–Bauer (KB) method. The inhibition zone was 24, 24, and 27 mm, respectively | [27] |
| Ag/CS | LiOH, KOH, CH4N2O, AgNO3, and Na3C6H5O7 | Spherical and ellipsoidal NPs; 4.45–9.22 nm | Chemical reduction with sodium citrate, in situ | E. coli and S. aureus | In vivo; rats | Antibacterial activity: 99.86% and 99.94%, respectively | [22] |
| Ag/CM- βCD | Chitosan, NaBH4, AgNO3, NaOH, cyclodextrin, CH₃CO2H, and C5H8O | 50 nm | Chemical reduction with NaBH4, in situ | E. coli and S. aureus | In vitro | The inhibition zone increased when the CM-βCD concentration was increased in the hydrogel | [25] |
| Ag/N, O-carboxymehtyl chitosan (N, O-CMC) | Chitosan, AgNO3, C10H16N2O8 (EDTA), CaCl2, FeCl3, and C2H3ClO2 | 25 nm | Chemical reduction using C2H3ClO2 | E. coli ATCC25922, S. aureus ATCC35556, MRSA ATCC 43300, P. aeruginosa ATCC47085, and K. pneumonia ATCC700603 | In vitro, L929 cells | MIC values: 48.5 mg/mL for P. aeruginosa; 32.0 mg/mL for S. aureus and MRSA; 17.5 mg/mL for E. coli, and 23.0 mg/mL for K. pneumonia | [37] |
| Ag/OKGM-CMCS | Oxidized konjac glucomannan (OKGM) and Carboxymethyl chitosan (CMCS) | 60 nm | Schiff-base reaction | S. aureus and E. coli | In vitro, L929 cells; In vivo, rats | The Ag/hydrogel achieved high antimicrobial activity, but the inhibition zone values were not displayed | [23] |
| Ag/KGM | Eggs, konjac glucomannan, AgNO3, and NaOH | 9.5–30.2 nm | In situ | S. aureus and E. coli | In vitro; In L929 cells; in vivo, rabbits | Good antibacterial efficiency on rabbits’ skin infections | [111] |
| Ag/CMC/PVA/EGDE | Carboxymethyl cellulose (CMC), polyvinyl alcohol (PVA), and ethylene glycol diglycidyl ether (EGDE) | 8–14 nm | Microwave radiation | E. coli, K. pneumoniae, P. aeruginosa, Proteus vulgaris, S. aureus, and Proteus mirabilis | In vitro, patient urine | The inhibition zone: 16.6 mm for E. coli, 15.8 mm for K. pneumoniae, 15.6 mm for P. aeruginosa and 15.2 mm for P. vulgaris | [13] |
| QCT-Ag/Carbopol- aloe vera | Carbopol 934, AgNO3, QCT, polyvinylpyrrolidone (PVP), Aloe vera, C3H8O3, and NaBH4 | 44.1 nm; ζ: −14.76 mV | Chemical reduction with NaBH4 | S. aureus MTCC 3160 and E. coli BL-21 | In vitro, L929 cells; In vivo, mice skin | The inhibition zone: 17 mm for E. coli and 19 mm for S. aureus | [63] |
| Ag/graphene | AgNO3, C7H10N2O2, (NH4)2S2O8, and NH3H2O | 39 nm | Hummer’s method | E. coli and S. aureus | In vitro, L929 cells; In vivo, rats | The disc diffusion method. Large Ag concentration led to great antibacterial activity using 5:1% wt. of Graphene | [41] |
| Ag/poly(vinyl alcohol)/chitosan/graphene | Graphene, chitosan, CH3CO2H, KNO3, AgNO3, and K2HPO4 | 6.38–10.00 nm | Electrochemical synthesis in situ using 90 V | E. coli ATCC 25922 and S. aureus TL | In vitro, MRC-5 and L929 cells; | The inhibition zone: 15.5 mm for S. aureus and 13.5 mm for E. coli; great antimicrobial activity with the 0.25Ag/PVA/0.5CHI/Gr | [70,71,72] |
| Ag/PEI- graphene oxide | Pluronic F 127, graphene oxide, C8H17N3.HCl, AgNO3, NH4OH, and NaCl | 10 nm; ζ: 42.6 mV | Amidation reaction with Ag(NH3)2OH by microwave reactor | E. coli and C. albicans | In vitro | E. coli (99.86%) and C. albicans (99.94%) | [66] |
| Ag/PAA-MBA | K2S2O8, NaBH4, PVP, C3H5NO, C6H9Na3O9, and AgNO3 | Spherical: 12.7 nm; triangular: 37.1 nm; hexagonal: 26.9 nm | Chemical reduction using NaBH4 | E. coli W3110 | In vitro | The spherical and triangular shapes of the Ag NPs displayed better antibacterial activity than the rod-shaped NPs. | [40] |
| Ag/halloysite/gelatin methacrylate | AgNO3, NaBH4, (CH3)2SO, and C2H4O | Ag NPs changed the microstructure and roughness of the hydrogel | In situ by photopolymerization using UV radiation (365 nm and 400 W) | E. coli ATCC 8739 and S. aureus ATCC 29213 | In vitro; In vivo, crania of rats | The inhibition zone test showed that the hydrogel restrained the growth of the bacteria | [65] |
| Ag/KGM | Chitosan, carboxymethyl, β-cyclodextrin, etc. | 50 nm | Chemical reduction | S. aureus and E. coli | In vitro | Inhibition zone: 22 and 19 mm, respectively | [25] |
| System | Materials | Au NP Properties (Size and Surface Charge) | NP Synthesis Method | Bacteria | Target | Antibacterial Properties: Inhibition Zone (mm) and MIC Values | Ref. |
|---|---|---|---|---|---|---|---|
| AuC/liposome | Cationic phospholipid liposomes, acrylamide, (glycol) dimethacrylate (PEGDMA) | 97.1 nm, ζ: −25.3 mV | Chemical reduction with NaBH4 | S. aureus MRSA252 | In vitro; in vivo, mice | No skin reaction after 7-day treatment. Hydrogel activity was influenced by pH | [133] |
| Au NSt/alginate | Sodium alginate (SA), CaCl2, and polyethylene imine (PEI) | Core diameter: 25 nm; Spikes size: 50 nm, 70 nm, and 120 nm | Chemical reduction with trisodium citrate | S. aureus MTCC1430 P. aeruginosa MTCC 1934 E. coli MTCC 443 | In vitro, NIH-3T3; in vivo, rats | The plate count method; the antimicrobial activity: 35.4% (S. aureus), and >80% (P. aeruginosa and E. coli.) | [126] |
| Au/poly (acrylamide-co-alginate) | Acrylamide (AM), alginate (SA), N,N-methylenebisacrylamide, and HAuCl4 | 8 nm | In situ, chemical reduction | E. coli | in vitro | Optical absorbance around 0.05–0.75. E. coli did not growth more after 2 h 30 min | [59] |
| CS-Au–MMT/gelatin | 2-mercapto-1-methylimidazole (MMT), tannin acid, chitosan (CS), and gelatin | 10.07 ± 2.34 nm 8.32 ± 1.97 nm | Chemical reduction | S. aureus ATCC 25923, E. coli ATCC 25922 MRSA | In vitro, L929 and L02; in vivo, rabbits | In situ; the microtiter broth dilution method, and MIC < 20 µM for all bacteria | [60] |
| Au-Ag/CS/TEOS | HAuCl4, HNO3, chitosan, and tetraethyl orthosilicate (TEOS) | Ag: 16 ± 25% nm Au: 19 ± 18% nm | Polymerization reaction and drop casting method | E. coli | In vitro | Crystal violet attachment; 80% inhibition of E. coli on the surface | [136] |
| Au–APA/gelatin | 6-aminopenicillanic acid (APA), gelatin, and HAuCl4, | 5 nm | Chemical reduction by NaBH4 | E. coli, K. pneumoniae, P. aeruginosa, MDR E. coli, and MDR K. pneumoniae | In vivo, rats | The microtiter broth dilution method; MIC were 2.5 µg/mL against E. coli and K. pneumoniae, >5 µg/mL against P. aeruginosa, 5 µg/mL against MDR E. coli and MDR K. pneumoniae | [61] |
| Au/HPMC | Tetrachloroauric acid, cetyltrimethyl ammonium bromide, ascorbic acid, NaBH4, AgNO3, and hydroxypropyl methylcellulose (HPMC) | 82.5 nm; ζ: 34.8 mV | Chemical reduction method using CTAB and NaBH4. Au NPs were embedded into HPMC | Staph. aureus ATCC 10400, E. coli ATCC 25922, and C. albicans ATCC 90028 | In vitro; in vivo, rats | Micro broth dilution assay. MIC and MBC: 0.25 and 0.1 nM/mL for Staph. aureus, MIC and MBC: 0.125 and 0.125 nM/mL for E. coli, and MIC and MBC: 0.25 and 0.5 nM/mL for C. albicans, | [24] |
| Au/Silk | HAuCl4, sodium citrate, bombyx mori cocoons, NaCO3, and LiBr | 13 nm | Chemical reduction using sodium citrate | E. coli ATCC 25922 and S. aureus ATCC 25923 | In vitro; in vivo, mice | Killed 80% of bacteria in 10 min; using a laser exposure time of 15 min and 600 mW, the zone inhibition was about 16 mm2 | [26] |
| Au/CA-DEG-IAA | Citric acid (CA), diethylene glycol (DEG), and indolylacetic acid (IAA) | 17 nm | In situ; chemical reduction with Na3C6H5O7 | S. aureus | In vitro | The diffusion method; Inhibition zone: 8.33–11.6 mm | [140] |
| Au/CA-DEG-IAA Ag/CA-DEG-IAA | Citric acid (CA), diethyleneglycol (DEG), and indole-3-acetic acid (IAA) | Au NPs: 8–30 nm Ag NPs: 4–12 nm | Condensation polycondensation; chemical reduction with Na3C6H5O7 | S. aureus, E. coli, and Bacillus cereus | In vitro | Inhibition zone (mm): 25 and 15 mm, 23 and 14 mm, 25 and 15 mm | [128] |
| Au/poloxamer 407 | CTAB (C16H33N(CH3)3Br), PAA (polyacrylic acid), PAH (poly(allylamine hydrochloride), and PEG (Poly(ethylene glycol) | Rod shape: 49.2 nm Spherical shape: 29.2 nm | Chemical reduction using Na3C6H5O7 | S. aureus ATCC 29213, and P. aeruginosa ATCC 27853 | In vitro; in vivo, rats | Reduction in bacterial viable count was >99.5% and 99.0> against S. aureus and P. aeruginosa using PAH-Au NPs and PEG-Au NPs. | [130] |
| FPAu | Polyethyleneimine (PEI), Polydopamine (PDA), Pluronic F127, 4-hydroxy benzaldehyde (PHBA), HAuCl4, and K2CO3 | 10 nm | Chemical reduction with NaBH4, and polyvinyl pyrrolidone (PVP) | E. coli S. aureus | In vitro; in vivo, rats | The plate count method; inhibited bacterial growth in 75% after 2 h | [68] |
| Au–PDA/PNAGA | HAuCl4, NaBH4, dopamine hydrochloride, and N-acryloyl glycinamide (PNAGA) | Diameter 32 nm and length 54 nm | Seeded growth method Polymerization | S. aureus ATCC29213 and E. coli ATCC25922 | In vitro, L929 cells; in vivo, rats | 97.6%; 98.4% | [131] |
| Ag–Au/carbopol | Carbopol® 980, acrylamide, AgNO3, and HAuCl4 | 2–8 nm | In situ reduction using mint leaf extract | Bacillus E. coli | In vitro | The disc method; inhibition zone: 18.5 mm 18.1 mm | [64] |
| Ag–Au/CMT | AgNO3, KAuCl4, and carboxy methyl tamarind (CMT) | 187 nm | Seeded growth method | Clinical E. cloacae isolate Ec18, E. cloacae BAA-1143, ATCC, and E. coli BAA-2469, ATCC | In vitro; in vivo, mice | The disc method. MIC: 6 µg/mL, 6 µg/mL, and 3 µg/mL | [29] |
| Au/Ag–gelatin | glutathione (GSH), HAuCl4, AgNO3, and N-hydroxysuccinimide (NHS) | Au NCs: 1.5–3.5 nm Au/Ag: 102 nm | Au/Ag NCs was incorporated into gelatin after NPs synthesis | P. aeruginosa | In vitro, pigskin | Inhibition zone: 31.9 mm | [144] |
| Au or Ag/silk fibroin | AgNO3, HAuCl4, and cocoons of Bombyx mori silkworm | Au NPs: 9–55 nm Ag NPs: 12–69 nm | In situ chemical reduction | S. aureus ATCC 33591, MRSA and P. aeruginosa ATCC 27853 S. aureus ATCC 25923, MSSA and E. coli ATCC 25922 S. epidermidis RP62A ATCC 35984. | In vitro, MG63 cells | Using sessile and planktonic bacteria. 0.1% of Au NPs were effective against S. aureus, and E. coli while 0.5% of Au NPs was antibacterial against P. aeruginosa | [28] |
| Au–ZIF8/OSA-GelMA | HAuCl, Na3C6H5O7, polyvinyl pyrrolidone (PVP), gelatin, Zn(NO3)2.6H2O, CH6N4O, oxidized sodium alginate (OSA), and carbohydrazide-modified methacrylated gelatin (GelMA-CDH) | 15 nm; ζ: −4.8 mV | Chemical reduction with Na3C6H5O7; Schiff-base reaction, and radical polymerization | E. coli ATCC 25922 S. aureus ATCC 29213 | In vitro, NIH-3 T3 cells | The number of bacteria colonies decreased by more than 99% | [69] |
| Au/C/PAM | Acrylamide monomer, cellulose, HAuCl4, and ciprofloxacin | Length: 5 µm and diameter 70 nm | In situ; chemical reduction with Na3C6H5O7 | E. coli, S. flexneri, Bacillus cereus, and Listeria inuaba | In vitro, L929 cells | The diffusion method; the antibacterial activity was 95% against the E. coli, and 79% against the S. flexneri. | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno Ruiz, Y.P.; de Almeida Campos, L.A.; Alves Agreles, M.A.; Galembeck, A.; Macário Ferro Cavalcanti, I. Advanced Hydrogels Combined with Silver and Gold Nanoparticles against Antimicrobial Resistance. Antibiotics 2023, 12, 104. https://doi.org/10.3390/antibiotics12010104
Moreno Ruiz YP, de Almeida Campos LA, Alves Agreles MA, Galembeck A, Macário Ferro Cavalcanti I. Advanced Hydrogels Combined with Silver and Gold Nanoparticles against Antimicrobial Resistance. Antibiotics. 2023; 12(1):104. https://doi.org/10.3390/antibiotics12010104
Chicago/Turabian StyleMoreno Ruiz, Yolice Patricia, Luís André de Almeida Campos, Maria Andressa Alves Agreles, André Galembeck, and Isabella Macário Ferro Cavalcanti. 2023. "Advanced Hydrogels Combined with Silver and Gold Nanoparticles against Antimicrobial Resistance" Antibiotics 12, no. 1: 104. https://doi.org/10.3390/antibiotics12010104
APA StyleMoreno Ruiz, Y. P., de Almeida Campos, L. A., Alves Agreles, M. A., Galembeck, A., & Macário Ferro Cavalcanti, I. (2023). Advanced Hydrogels Combined with Silver and Gold Nanoparticles against Antimicrobial Resistance. Antibiotics, 12(1), 104. https://doi.org/10.3390/antibiotics12010104