Photoinactivation of Planktonic Cells, Pseudohyphae, and Biofilms of Candida albicans Sensitized by a Free-Base Chlorin and Its Metal Complexes with Zn(II) and Pd(II)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spectroscopic Measurements
2.2. Photooxidation of DMA
2.3. Strains and Cultures of C. albicans
2.4. Experiments in C. albicans Planktonic Cells
2.5. Growth Curves of C. albicans
2.6. Studies in C. albicans Pseudohyphae
2.7. Tests in C. albicans Biofilms
2.8. Controls and Statistical Analysis
3. Results and Discussion
3.1. Molecular Structure of Chlorin Derivatives
3.2. Spectroscopic Characterization
3.3. Photooxidation of DMA and O2(1Δg) Formation
3.4. Photokilling of C. albicans Planktonic Cells
3.5. Photodynamic Mechanism in C. albicans Cells
3.6. Photoinactivation of C. albicans Cells under Growth Conditions
3.7. Photoinactivation of C. albicans Pseudohyphae
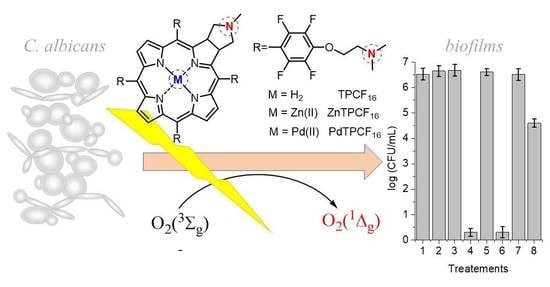
3.8. Photokilling of C. albicans Biofilms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Wang, X.; Li, R. Cutaneous and subcutaneous fungal infections: Recent developments on host-fungus interactions. Curr. Opin. Microbiol. 2021, 62, 93–102. [Google Scholar] [CrossRef]
- Bankaitis, V.A.; Tripathi, A.; Chen, X.-R.; Igumenova, T.I. New strategies for combating fungal infections: Inhibiting inositol lipid signaling by targeting Sec14 phosphatidylinositol transfer proteins. Adv. Biol. Regul. 2022, 84, 100891. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Gaffen, S.L.; Hube, B. Candidalysin: Discovery and function in Candida albicans infections. Curr. Opin. Microbiol. 2019, 52, 100–109. [Google Scholar] [CrossRef]
- Wijnants, S.; Vreys, J.; Van Dijck, P. Interesting antifungal drug targets in the central metabolism of Candida albicans. Trends Pharmacol. Sci. 2022, 43, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Arita, G.S.; Faria, D.R.; Capoci, I.R.G.; Kioshima, E.S.; Bonfim-Mendonça, P.S.; Svidzinski, T.I.E. Cell wall associated proteins involved in filamentation with impact on the virulence of Candida albicans. Microbiol. Res. 2022, 258, 126996. [Google Scholar] [CrossRef]
- Wall, G.; Montelongo-Jauregui, D.; Bonifacio, B.V.; Lopez-Ribot, J.L.; Uppuluri, P. Candida albicans biofilm growth and dispersal: Contributions to pathogenesis. Curr. Opin. Microbiol. 2019, 52, 1–6. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Morace, G.; Perdoni, F.; Borghi, E. Antifungal drug resistance in Candida species. J. Glob. Antimicrob. Resist. 2014, 2, 254–259. [Google Scholar] [CrossRef]
- Robbins, N.; Cowen, L.E. Antifungal discovery. Curr. Opin. Microbiol. 2022, 69, 102198. [Google Scholar] [CrossRef]
- Capoor, M.R.; Subudhi, C.P.; Collier, A.; Bal, A.M. Antifungal stewardship with an emphasis on candidaemia. J. Glob. Antimicrob. Resist. 2019, 19, 262–268. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial photodynamic therapy: Latest developments with a focus on combinatory strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Durantini, A.M.; Heredia, D.A.; Durantini, J.E.; Durantini, E.N. BODIPYs to the rescue: Potential applications in photodynamic inactivation. Eur. J. Med. Chem. 2018, 144, 651–661. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Liu, Y.-C.; Sun, H.; Guo, D.-S. Type I photodynamic therapy by organic-inorganic hybrid materials: From strategies to applications. Coord. Chem. Rev. 2019, 395, 46–62. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Durantini, J.E.; Durantini, E.N. Fullerene C60 derivatives as antimicrobial photodynamic agents. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100471. [Google Scholar] [CrossRef]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updates 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Souza, T.H.S.; Sarmento-Neto, J.F.; Souza, S.O.; Raposo, B.L.; Silva, B.P.; Borges, C.P.F.; Santos, B.S.; Cabral Filho, P.E.; Rebouças, J.S.; Fontes, A. Advances on antimicrobial photodynamic inactivation mediated by Zn(II) porphyrins. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100454. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, J.E.; Ferreyra, D.D.; Reynoso, E.; Gonzalez Lopez, E.J.; Durantini, A.M.; Milanesio, M.E.; Durantini, E.N. Charge density distribution effect in pyrrolidine-fused chlorins on microbial uptake and antimicrobial photoinactivation of microbial pathogens. J. Photochem. Photobiol. B Biol. 2021, 225, 112321. [Google Scholar] [CrossRef]
- Costa, D.C.S.; Gomes, M.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Cavaleiro, J.A.S.; Almeida, A.; Tomé, J.P.C. Comparative photodynamic inactivation of antibiotic resistant bacteria by first and second generation cationic photosensitizers. Photochem. Photobiol. Sci. 2012, 11, 1905–1913. [Google Scholar] [CrossRef]
- Santos, I.; Gamelas, S.R.D.; Vieira, C.; Faustino, M.A.F.; Tomé, J.P.C.; Almeida, A.; Gomes, A.T.P.C.; Lourenço, L.M.O. Pyrazole-pyridinium porphyrins and chlorins as powerful photosensitizers for photoinactivation of planktonic and biofilm forms of E. coli. Dyes Pigment. 2021, 193, 109557. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Synthesis, spectroscopic properties and photodynamic activity of porphyrinefullerene C60 dyads with application in the photodynamic inactivation of Staphylococcus aureus. Eur. J. Med. Chem. 2014, 83, 685–694. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Milanesio, M.E.; Fujita, H.; Lindsey, J.S.; Durantini, E.N. Bacteriochlorin-bis(spermine) conjugate affords an effective photodynamic action to eradicate microorganisms. J. Biophotonics 2020, 13, e201960061. [Google Scholar] [CrossRef] [PubMed]
- Cormick, M.P.; Alvarez, M.G.; Rovera, M.; Durantini, E.N. Photodynamic inactivation of Candida albicans sensitized by tri- and tetra-cationic porphyrin derivatives. Eur. J. Med. Chem. 2009, 44, 1592–1599. [Google Scholar] [CrossRef]
- Quiroga, E.D.; Cordero, P.; Mora, S.J.; Alvarez, M.G.; Durantini, E.N. Mechanistic aspects in the photodynamic inactivation of Candida albicans sensitized by a dimethylaminopropoxy porphyrin and its equivalent with cationic intrinsic charges. Photodiagn. Photodyn. Ther. 2020, 31, 101877. [Google Scholar] [CrossRef] [PubMed]
- Agazzi, M.L.; Durantini, J.E.; Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. A novel tricationic fullerene C60 as broad-spectrum antimicrobial photosensitizer: Mechanisms of action and potentiation with potassium iodide. Photochem. Photobiol. Sci. 2021, 20, 327–341. [Google Scholar] [CrossRef]
- Quiroga, E.D.; Mora, S.J.; Alvarez, M.G.; Durantini, E.N. Photodynamic inactivation of Candida albicans by a tetracationictentacle porphyrin and its analogue without intrinsic charges inpresence of fluconazole. Photodiagn. Photodyn. Ther. 2016, 13, 334–340. [Google Scholar] [CrossRef]
- Samaranayake, Y.H.; Cheung, B.P.K.; Yau, J.Y.Y.; Yeung, S.K.W.; Samaranayake, L.P. Human serum promotes Candida albicans biofilm growth and virulence gene expression on silicone biomaterial. PLoS ONE 2013, 8, e62902. [Google Scholar] [CrossRef] [Green Version]
- Cordero, P.V.; Ferreyra, D.D.; Pérez, M.E.; Alvarez, M.G.; Durantini, E.N. Photodynamic Effect of 5,10,15,20-Tetrakis[4-(3-N,N-dimethylaminopropoxy) phenyl]chlorin towards the human pathogen Candida albicans under different culture conditions. Photochem 2021, 1, 505–522. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Sarotti, A.M.; Gsponer, N.S.; Ferreyra, D.D.; Bertolotti, S.G.; Milanesio, M.E.; Durantini, E.N. Proton-dependent switching of a novel amino chlorin derivative as a fluorescent probe and photosensitizer for acidic media. Chem. Eur. J. 2018, 24, 5950–5961. [Google Scholar] [CrossRef]
- Milanesio, M.E.; Alvarez, M.G.; Bertolotti, S.G.; Durantini, E.N. Photophysical characterization and photodynamic activity of metallo 5-(4-(trimethylammonium)phenyl)-10,15,20-tris(2,4,6-trimethoxyphenyl)porphyrin in homogeneous and biomimetic media. Photochem. Photobiol. Sci. 2008, 7, 963–972. [Google Scholar] [CrossRef]
- Hirohara, S.; Kawasaki, Y.; Funasako, R.; Yasui, N.; Totani, M.; Alitomo, H.; Yuasa, J.; Kawai, T.; Oka, C.; Kawaichi, M.; et al. Sugar and heavy atom effects of glycoconjugated chlorin palladium complex on photocytotoxicity. Bioconjug. Chem. 2012, 23, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Zawadzka, M. Structural investigation of 5,10-A2B2-type porphyrins: Palladium(II) and zinc(II) complexes of 5,10-dibromo-15,20-bis(4-methylphenyl)porphyrin. Acta Crystallogr. C Struct. Chem. 2014, 70, 1143–1146. [Google Scholar] [CrossRef] [Green Version]
- McGill, S.; Nesterov, V.N.; Gould, S.L. [5,10,15,20-Tetrakis(4-methoxyphenyl)-porphyrinato]zinc dichloromethane disolvate. Acta Crystallogr. E Crystallogr. Commun. 2013, 70, m470. [Google Scholar] [CrossRef] [Green Version]
- Obata, M.; Hirohara, S.; Tanaka, R.; Kinoshita, I.; Ohkubo, K.; Fukuzumi, S.; Tanihara, M.; Yano, S. In vitro heavy-atom effect of palladium(II) and platinum(II) complexes of pyrrolidine-fused chlorin in photodynamic therapy. J. Med. Chem. 2009, 52, 2747–2753. [Google Scholar] [CrossRef]
- Almeida, J.; Silva, A.M.N.; Rebelo, S.L.H.; Cunha-Silva, L.; Rangel, M.; de Castro, B.; Leite, A.; Silva, A.M.G. Synthesis and coordination studies of 5-(40-carboxyphenyl)-10,15,20-tris(pentafluorophenyl)porphyrin and its pyrrolidine-fused chlorin derivative. New J. Chem. 2018, 42, 8169–8179. [Google Scholar] [CrossRef]
- Ferreyra, D.D.; Reynoso, E.; Cordero, P.; Spesia, M.B.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Synthesis and properties of 5,10,15,20-tetrakis[4-(3-N,N-dimethylaminopropoxy)phenyl]chlorin as potential broad-spectrum antimicrobial photosensitizers. J. Photochem. Photobiol. B Biol. 2016, 158, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Novaira, M.; Cormick, M.P.; Durantini, E.N. Spectroscopic and time-resolved fluorescence emission properties of a cationic and an anionic porphyrin in biomimetic media and Candida albicans cells. J. Photochem. Photobiol. A Chem. 2012, 246, 67–74. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Heredia, D.A.; Martínez, S.R.; Durantini, A.M.; Pérez, M.E.; Mangione, M.I.; Durantini, J.E.; Gervaldo, M.A.; Otero, L.A.; Durantini, E.N. Antimicrobial photodynamic polymeric films bearing biscarbazol triphenylamine end-capped dendrimeric Zn(II) porphyrin. ACS Appl. Mater. Interfaces 2019, 11, 27574–27587. [Google Scholar] [CrossRef]
- da Silva, E.F.F.; Pedersen, B.W.; Breitenbach, T.; Toftegaard, R.; Kuimova, M.K.; Arnaut, L.G.; Ogilby, P.R. Irradiation- and sensitizer-dependent changes in the lifetime of intracellular singlet oxygen produced in a photosensitized process. J. Phys. Chem. B 2012, 116, 445–461. [Google Scholar] [CrossRef]
- Cormick, M.P.; Quiroga, E.D.; Bertolotti, S.G.; Alvarez, M.G.; Durantini, E.N. Mechanistic insight of the photodynamic effect induced by tri- and tetra-cationic porphyrins on Candida albicans cells. Photochem. Photobiol. Sci. 2011, 10, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, M.A.; Alvarez, M.G.; Durantini, E.N. Photodynamic action mechanism mediated by zinc(II) 2,9,16,23-tetrakis [4-(N-methylpyridyloxy)]phthalocyanine in Candida albicans cells. Photochem. Photobiol. 2015, 91, 1203–1209. [Google Scholar] [CrossRef]
- Gsponer, N.S.; Agazzi, M.L.; Spesia, M.B.; Durantini, E.N. Approaches to unravel pathways of reactive oxygen species in the photoinactivation of bacteria induced by a dicationic fulleropyrrolidinium derivative. Methods 2016, 109, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Involvement of type I and type II mechanisms on the photoinactivation of non-enveloped DNA and RNA bacteriophages. J. Photochem. Photobiol. B Biol. 2013, 120, 10–16. [Google Scholar] [CrossRef]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Porphyrins containing basic aliphatic amino groups as potential broad spectrum antimicrobial agents. Photodiagn. Photodyn. Ther. 2018, 24, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. Susceptibility of Candida albicans to photodynamic action of 5,10,15,20-tetra(4-N-methylpyridyl)porphyrin in different media. FEMS Immunol. Med. Microbiol. 2010, 60, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, M.; Wang, Y.; Chen, Y.; Gao, J.; Ying, C. ERG11 couples oxidative stress adaptation, hyphal elongation and virulence in Candida albicans. FEMS Yeast Res. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Jackson, Z.; Meghji, S.; MacRobert, A.; Henderson, B.; Wilson, M. Killing of the yeast and hyphal forms of Candida albicans using a light-activated antimicrobial agent. Lasers Med. Sci. 1999, 14, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Bliss, J.M.; Bigelow, C.E.; Foster, T.H.; Haidaris, C.G. Susceptibility of Candida species to photodynamic effects of Photofrin. Antimicrob. Agents Chemother. 2004, 48, 2000–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swidergall, M. Candida albicans at host barrier sites: Pattern recognition receptors and beyond. Pathogens 2019, 8, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciurea, C.N.; Kosovski, I.B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and candidiasis-opportunism versus pathogenicity: A review of the virulence traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef]
- Duarte-Peña, L.; López-Saucedo, F.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Modification of indwelling PVC catheters by ionizing radiation with temperature- and pH-responsive polymers for antibiotic delivery. Radiat. Phys. Chem. 2022, 193, 110005. [Google Scholar] [CrossRef]
- Pinto, A.P.; Bueno Rosseti, I.; Lopes Carvalho, M.; Graziele Marques da Silva, B.; Alberto-Silva, C.; Silva Costa, M. Photodynamic Antimicrobial Chemotherapy (PACT), using Toluidine blue O inhibits the viability of biofilm produced by Candida albicans at different stages of development. Photodiagn. Photodyn. Ther. 2018, 21, 182–189. [Google Scholar] [CrossRef]
- Shi, H.; Li, J.; Zhang, H.; Zhang, J.; Sun, H. Effect of 5-aminolevulinic acid photodynamic therapy on Candida albicans biofilms: An in vitro study. Photodiagn. Photodyn. Ther. 2016, 15, 40–45. [Google Scholar] [CrossRef]
- Shi, H.; Li, J.; Peng, C.; Xu, B.; Sun, H. The inhibitory activity of 5-aminolevulinic acid photodynamic therapy (ALA-PDT) on Candida albicans biofilms. Photodiagn. Photodyn. Ther. 2021, 34, 102271. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Trivellin, N.; Mat’átková, O.; Paldrychová, M.; Baj, A.; Caruso, E. Photodynamic therapy by diaryl-porphyrins to control the growth of Candida albicans. Cosmetics 2020, 7, 31. [Google Scholar] [CrossRef]
- Souza, S.O.; Raposo, B.L.; Sarmento-Neto, J.F.; Rebouças, J.S.; Macêdo, D.P.C.; Figueiredo, R.C.B.Q.; Santos, B.S.; Freitas, A.Z.; Cabral Filho, P.E.; Ribeiro, M.S.; et al. Photoinactivation of yeast and biofilm communities of Candida albicans mediated by ZnTnHex-2-PyP4+ porphyrin. J. Fungi 2022, 8, 556. [Google Scholar] [CrossRef]
- Vieira, C.; Bartolomeu, M.; Santos, A.R.; Mesquita, M.Q.; Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Photoinactivation of bacterial and fungal planktonic/biofilm forms using the combination of a porphyrinic formulation with potassium iodide. Med. Sci. Forum 2022, 12, 13. [Google Scholar]
- Milanesio, M.E.; Alvarez, M.G.; Yslas, E.I.; Borsarelli, C.D.; Silber, J.J.; Rivarola, V.; Durantini, E.N. Photodynamic studies of metallo 5,10,15,20-tetrakis(4-methoxyphenyl) porphyrin: Photochemical characterization and biological consequences in a human carcinoma cell line. Photochem. Photobiol. 2001, 74, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.E.; Durantini, J.E.; Reynoso, E.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Porphyrin-schiff base conjugates bearing basic amino groups as antimicrobial phototherapeutic agents. Molecules 2021, 26, 5877. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, S.C.; Heredia, D.A.; Durantini, A.M.; Durantini, E.N. Antimicrobial photosensitizing material based on conjugated Zn(II) porphyrins. Antibiotics 2022, 11, 91. [Google Scholar] [CrossRef] [PubMed]












| PS | λSoret (nm) | εSoret a | λQI (nm) | εQI a | λem (nm) | ΦF b |
|---|---|---|---|---|---|---|
| TPCF16 | 408 | 1.5 × 105 | 651 | 3.5 × 104 | 653 | 0.11 ± 0.01 c |
| ZnTPCF16 | 418 | 1.7 × 105 | 621 | 2.9 × 104 | 626 | 0.040 ± 0.002 |
| PdTPCF16 | 405 | 1.6 × 105 | 602 | 5.7 × 104 | - | - |
| PS | kobsDMA (s−1) a | ΦΔ b |
|---|---|---|
| TPCF16 | - | 0.34 ± 0.02 c |
| ZnTPCF16 | (5.81 ± 0.05) × 10−4 | 0.71 ± 0.03 d |
| PdTPCF16 | (6.15 ± 0.07) × 10−4 | 0.75 ± 0.04 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordero, P.V.; Alvarez, M.G.; Gonzalez Lopez, E.J.; Heredia, D.A.; Durantini, E.N. Photoinactivation of Planktonic Cells, Pseudohyphae, and Biofilms of Candida albicans Sensitized by a Free-Base Chlorin and Its Metal Complexes with Zn(II) and Pd(II). Antibiotics 2023, 12, 105. https://doi.org/10.3390/antibiotics12010105
Cordero PV, Alvarez MG, Gonzalez Lopez EJ, Heredia DA, Durantini EN. Photoinactivation of Planktonic Cells, Pseudohyphae, and Biofilms of Candida albicans Sensitized by a Free-Base Chlorin and Its Metal Complexes with Zn(II) and Pd(II). Antibiotics. 2023; 12(1):105. https://doi.org/10.3390/antibiotics12010105
Chicago/Turabian StyleCordero, Paula V., María G. Alvarez, Edwin J. Gonzalez Lopez, Daniel A. Heredia, and Edgardo N. Durantini. 2023. "Photoinactivation of Planktonic Cells, Pseudohyphae, and Biofilms of Candida albicans Sensitized by a Free-Base Chlorin and Its Metal Complexes with Zn(II) and Pd(II)" Antibiotics 12, no. 1: 105. https://doi.org/10.3390/antibiotics12010105
APA StyleCordero, P. V., Alvarez, M. G., Gonzalez Lopez, E. J., Heredia, D. A., & Durantini, E. N. (2023). Photoinactivation of Planktonic Cells, Pseudohyphae, and Biofilms of Candida albicans Sensitized by a Free-Base Chlorin and Its Metal Complexes with Zn(II) and Pd(II). Antibiotics, 12(1), 105. https://doi.org/10.3390/antibiotics12010105