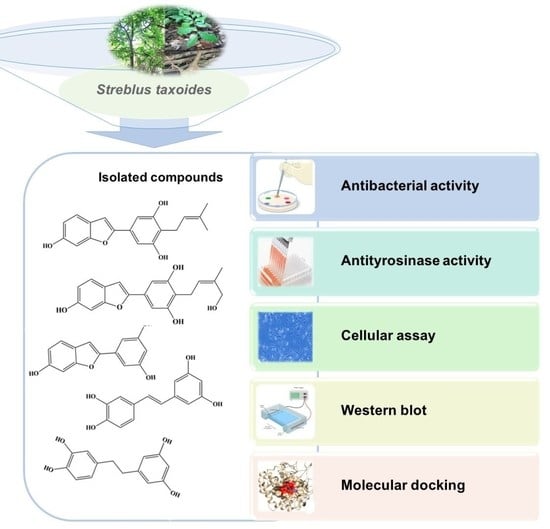
Chemical Constituents from Streblus taxoides Wood with Their Antibacterial and Antityrosinase Activities Plus in Silico Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Isolated Compounds
2.2. Antimicrobial Activity
2.3. Enzymatic Antityrosinase Activity
2.4. Cell Viability
2.5. Intracellular Antityrosinase Activity and Melanin Content
2.6. Western Blot
2.7. Molecular Docking Experiment
3. Materials and Methods
3.1. Plant Materials
3.2. Extraction and Isolation
3.3. Spectroscopic Data
- (1)
- ω-Hydroxymoracin C; C19H18O5
- (2)
- Moracin M; C14H10O4 [41]
- (3)
- Moracin C; C19H18O4 [60]
- (4)
- 3, 4, 3′, 5′-Tetrahydroxybibenzyl; C14H14O4 [61]
- (5)
- Piceatannol; C14H12O4 [62]
3.4. Antimicrobial Activity Assay
3.5. Enzymetic Antityrosinase Activity Assay
3.6. Cell Culture
3.7. Cell Viability Assay
3.8. Intracellular Antityrosinase Activity and Melanin Content Assays
3.9. Western Blot Analysis
3.10. Molecular Docking Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Drott, J.B.; Alexeyev, O.; Bergstrom, P.; Elgh, F.; Olsson, J. Propionibacterium acnes infection induces upregulation of inflammatory genes and cytokine secretion in prostate epithelial cells. BMC Microbiol. 2010, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Jayaveera, K.N.; Kumar, C.K.A.; Sanjay, U.P.; Swamy, B.M.V.; Kumar, D.V.K. Antimicrobial effects of Indian medicinal plants against acne-inducing bacteria. J. Pharm. Res. 2007, 6, 717–723. [Google Scholar] [CrossRef]
- Athikomkulchai, S.; Watthanachaiyingcharoen, R.; Tunvichien, S.; Vayumhasuwan, P.; Karnsomkiet, P.; Sae-Jong, P.; Ruangrungsi, N. The development of anti-acne products from Eucalyptus globulus and Psidium guajava oil. J. Health Res. 2008, 22, 109–113. [Google Scholar]
- Swope, V.B.; Abdel-Malek, Z.; Kassem, L.M.; Nordlund, J.J. Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J. Investig. Dermatol. 1991, 96, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Piérard, G.E. Skin-lightening products revisited. Int. J. Cosmet. Sci. 2003, 25, 169–181. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, H.; Han, J.; Jin, S.H.; Park, J.Y.; Shin, D.W.; Lee, T.R.; Kim, K.; Lee, A.Y.; Noh, M. IL-4 inhibits the melanogenesis of normal human melanocytes through the JAK2-STAT6 signaling pathway. J. Investig. Dermatol. 2013, 133, 528–536. [Google Scholar] [CrossRef]
- Hossain, M.R.; Ansary, T.M.; Komine, M.; Ohtsuki, M. Diversified stimuli-induced inflammatory pathways cause skin pigmentation. Int. J. Mol. Sci. 2021, 22, 3970. [Google Scholar] [CrossRef]
- Fu, C.; Chen, J.; Lu, J.; Yi, L.; Tong, X.; Kang, L.; Pei, S.; Ouyang, Y.; Jiang, L.; Ding, Y.; et al. Roles of inflammation factors in melanogenesis (Review). Mol. Med. Rep. 2020, 21, 1421–1430. [Google Scholar] [CrossRef]
- Meng, X.; Yang, Y.; Wu, Y.; Zhang, Y.; Zhang, H.; Zhou, W.; Guo, M.; Li, L. Inflammatory factor expression in HaCaT cells and melanin synthesis in melanocytes: Effects of Ganoderma lucidum fermentation broth containing Chinese medicine. Int. J. Food Prop. 2022, 25, 1604–1621. [Google Scholar] [CrossRef]
- Yoshida, M.; Takahashi, Y.; Inoue, S. Histamine induces melanogenesis and morphologic changes by protein kinase A activation via H2 receptors in human normal melanocytes. J. Investig. Dermatol. 2000, 114, 334–342. [Google Scholar] [CrossRef]
- Jimenez-Cervantes, C.; Garcia-Borron, J.C.; Lozano, J.A.; Solano, F. Effect of detergents and endogenous lipids on the activity and properties of tyrosinase and its related proteins. Biochim. Biophys. Acta (BBA) 1995, 1243, 421–430. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Niu, C.; Aisa, H.A. Upregulation of melanogenesis and tyrosinase activity: Potential agents for vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [PubMed]
- Taweechaisupapong, S.; Wongkham, S.; Chareonsuk, S.; Suparee, S.; Srilalai, P.; Chaiyarak, S. Selective activity of Streblus asper on Mutans streptococci. J. Ethnopharmacol. 2000, 70, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.Y.; Son, K.H.; Kwon, C.S.; Kwon, G.S.; Kang, S.S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent., Sophora flavescens Ait. and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Kaitou, K.; Terada, S. Antimicrobial activity of 2-arylbenzofurans from Morus species against methicillin-resistant Staphylococcus aureus. Fitoterapia 2005, 76, 708–711. [Google Scholar] [CrossRef]
- Taweechaisupapong, S.; Choopan, T.; Singhara, S.; Chatrchaiwiwatana, S.; Wongkham, S. In vitro inhibitory effect of Streblus asper leaf-extract on adhesion of Candida albicans to human buccal epithelial cells. J. Ethnopharmacol. 2005, 96, 221–226. [Google Scholar] [CrossRef]
- Taweechaisupapong, S.; Klanrit, P.; Singhara, S.; Pitiphat, W.; Wongkham, S. Inhibitory effect of Streblus asper leaf-extract on adhesion of Candida albicans to denture acrylic. J. Ethnopharmacol. 2006, 106, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Kummee, S.; Intaraksa, N. Antimicrobial activity of Desmos chinensis leaf and Maclura cochinchinensis wood extracts. Songklanakarin J. Sci. Technol. 2008, 30, 635–639. [Google Scholar]
- Pandey, A.; Bhatnagar, S.P. Preliminary phytochemical screening and antimicrobial studies on Artocarpus lakoocha Roxb. Anc. Sci. Life 2009, 28, 21–24. [Google Scholar] [PubMed]
- Sohn, H.Y.; Kwon, C.S.; Son, K.H. Fungicidal effect of prenylated flavonol, papyriflavonol A, isolated from Broussonetia papyrifera (L.) Vent. against Candida albicans. J. Microbiol. Biotechnol. 2010, 20, 1397–1402. [Google Scholar] [CrossRef]
- Hashim, N.M.; Rahmani, M.; Ee, G.C.; Sukari, M.A.; Yahayu, M.; Amin, M.A.; Ali, A.M.; Go, R. Antioxidant, antimicrobial and tyrosinase inhibitory activities of xanthones isolated from Artocarpus obtusus F.M. Jarrett. Molecules 2012, 17, 6071–6082. [Google Scholar] [CrossRef]
- Tsai, P.W.; de Castro-Cruz, K.A.; Shen, C.-C.; Ragasa, C.Y. Chemical constituents of Broussonetia luzonicus. Pharmacogn. J. 2012, 4, 1–4. [Google Scholar] [CrossRef]
- Kamal, T.; Muzammil, A.; Omar, M.N. Evaluation of antimicrobial activity of Artocarpus altilis on pathogenic microorganisms. Sci. Ser. Data Rep. 2012, 4, 41–48. [Google Scholar]
- Lamounier, K.C.; Cunha, L.C.; de Morais, S.A.; de Aquino, F.J.; Chang, R.; de Nascimento, E.A.; de Souza, M.G.; Martins, C.H.; Cunha, W.R. Chemical analysis and study of phenolics, antioxidant activity, and antibacterial effect of the wood and bark of Maclura tinctoria (L.) D. Don ex Steud. Evid.-Based Complement. Altern. Med. 2012, 2012, 451039. [Google Scholar] [CrossRef]
- Pradhan, C.; Mohanty, M.; Rout, A. Phytochemical screening and comparative bioefficacy assessment of Artocarpus altilis leaf extracts for antimicrobial activity. Front. Life Sci. 2012, 6, 71–76. [Google Scholar] [CrossRef]
- Dharmaratne, H.R.W.; Jacob, M.; Tekwani, B.L.; Nanayakkara, N.P.D. Antimicrobial and antileishmanial compounds from Maclura pomifera fruits. Planta Med. 2013, 79, PF1. [Google Scholar] [CrossRef]
- Madhavi, Y.; Rao, D.B.; Rao, T.R. Studies on phytochemical analysis and antimicrobial activity of Artocarpus communis fruit latex against selected pathogenic microorganisms. Indo Am. J. Pharm. Res. 2013, 3, 1458–1468. [Google Scholar]
- Swargiary, A.; Ronghang, B. Screening of phytochemicals constituents, antioxidannt and antibacterial properties of methanolic bark extracts of Maclura cochinchinensis (Lour.) Corner. Int. J. Pharma Bio Sci. 2013, 4, 449–459. [Google Scholar]
- Dej-adisai, S.; Meechai, I.; Puripattanavong, J.; Kummee, S. Antityrosinase and antimicrobial activities from Thai medicinal plants. Arch. Pharmacal Res. 2014, 37, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Jamil, S.; Lathiff, S.M.A.; Abdullah, S.A.; Jemaon, N.; Sirat, H.M. Antimicrobial flavonoids from Artocarpus anisophyllus Miq. and Artocarpus lowii King. J. Teknol. 2014, 71, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Llagas, M.C.D.L.; Santiago, L.; Ramos, J.D. Antibacterial activity of crude ethanolic extract and solvent fractions of Ficus pseudopalma Blanco leaves. Asian Pac. J. Trop. Dis. 2014, 4, 367–371. [Google Scholar] [CrossRef]
- Teanpaisan, R.; Senapong, S.; Puripattanavong, J. In vitro antimicrobial and activity of Artocarpus lakoocha (Moraceae) extract against some oral pathogens. Trop. J. Pharm. Res. 2014, 13, 1149–1155. [Google Scholar] [CrossRef]
- Fongang, Y.S.F.; Bankeu, J.J.K.; Ali, M.S.; Awantu, A.F.; Zeeshan, A.; Assob, C.N.; Mehreen, L.; Lenta, B.N.; Ngouela, S.A.; Tsamo, E. Flavonoids and other bioactive constituents from Ficus thonningii Blume (Moraceae). Phytochem. Lett. 2015, 11, 139–145. [Google Scholar] [CrossRef]
- Weli, A.M.; Al-Blushi, A.A.M.; Hossain, M.A. Evaluation of antioxidant and antimicrobial potential of different leaves crude extracts of Omani Ficus carica against food borne pathogenic bacteria. Asian Pac. J. Trop. Dis. 2015, 5, 13–16. [Google Scholar] [CrossRef]
- Yessoufou, K.; Elansary, H.O.; Mahmoud, E.A.; Skalicka-Woźniak, K. Antifungal, antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Ind. Crop. Prod. 2015, 74, 752–758. [Google Scholar] [CrossRef]
- Dej-adisai, S.; Parndaeng, K.; Wattanapiromsakul, C. Determination of phytochemical compounds, and tyrosinase inhibitory and antimicrobial activities of bioactive compounds from Streblus ilicifolius (S Vidal) Corner. Trop. J. Pharm. Res. 2016, 15, 497–506. [Google Scholar] [CrossRef]
- Dej-adisai, S.; Parndaeng, K.; Wattanapiromsakul, C.; Nuankaew, W.; Kang, T.H. Effects of selected moraceae plants on tyrosinase enzyme and melanin content. Pharmacogn. Mag. 2019, 15, 708–714. [Google Scholar] [CrossRef]
- Dej-adisai, S.; Parndaeng, K.; Wattanapiromsakul, C.; Hwang, J.S. Three new isoprenylated flavones from Artocarpus chama stem and their bioactivities. Molecules 2021, 27, 3. [Google Scholar] [CrossRef] [PubMed]
- Sritularak, B. Chemical Constituents of Artocarpus lakoocha and A. gomezianus. Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 1998. [Google Scholar]
- Likhitwitayawuid, K.; Sritularak, B.; De-Eknamkul, W. Tyrosinase inhibitors from Artocarpus gomezianus. Planta Med. 2000, 66, 275–277. [Google Scholar] [CrossRef]
- Shimizu, K.; Kondo, R.; Sakai, K. Inhibition of tyrosinase by flavonoids, stilbenes and related 4-substituted resorcinols: Structure-activity investigations. Planta Med. 2000, 66, 11–15. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K.; Sornsute, A.; Sritularak, B.; Ploypradith, P. Chemical transformations of oxyresveratrol (trans-2,4,3′,5′-tetrahydroxystilbene) into a potent tyrosinase inhibitor and a strong cytotoxic agent. Bioorg. Med. Chem. Lett. 2006, 16, 5650–5653. [Google Scholar] [CrossRef] [PubMed]
- Tengamnuay, P.; Pengrungruangwong, K.; Pheansri, I.; Likhitwitayawuid, K. Artocarpus lakoocha heartwood extract as a novel cosmetic ingredient: Evaluation of the in vitro anti-tyrosinase and in vivo skin whitening activities. Int. J. Cosmet. Sci. 2006, 28, 269–276. [Google Scholar] [CrossRef]
- Ko, H.H.; Chang, W.L.; Lu, T.M. Antityrosinase and antioxidant effects of ent-kaurane diterpenes from leaves of Broussonetia papyrifera. J. Nat. Prod. 2008, 71, 1930–1933. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Cheng, K.W.; Chao, J.; Wu, J.; Wang, M. Tyrosinase inhibitors from paper mulberry (Broussonetia papyrifera). Food Chem. 2008, 106, 529–535. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Cheng, K.W.; To, J.T.; Li, H.; Wang, M. Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent. Mol. Nutr. Food Res. 2008, 52, 1530–1538. [Google Scholar] [CrossRef]
- Baek, Y.S.; Ryu, Y.B.; Curtis-Long, M.J.; Ha, T.J.; Rengasamy, R.; Yang, M.S.; Park, K.H. Tyrosinase inhibitory effects of 1,3-diphenylpropanes from Broussonetia kazinoki. Bioorg. Med. Chem. 2009, 17, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.P.; Chen, S.; Wang, S.; Wang, X.C.; Cheng, K.W.; Wu, J.J.; Yang, D.; Wang, M. Chemical components and tyrosinase inhibitors from the twigs of Artocarpus heterophyllus. J. Agric. Food Chem. 2009, 57, 6649–6655. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Yim, E.Y.; Song, G.; Lee, N.H.; Hyun, C.G. Screening of elastase and tyrosinase inhibitory activity from Jeju island plants. EurAsian J. Biosci. 2010, 4, 41–53. [Google Scholar] [CrossRef]
- Chang, L.W.; Juang, L.J.; Wang, B.S.; Wang, M.Y.; Tai, H.M.; Hung, W.J.; Chen, Y.J.; Huang, M.H. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem. Toxicol. 2011, 49, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and synthetic tyrosinase inhibitors as antibrowning agents: An update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Kang, Y.; Choi, J.-U.; Lee, E.-A.; Park, H.-R. Flaniostatin, a new isoflavonoid glycoside isolated from the leaves of Cudrania tricuspidata as a tyrosinase inhibitor. Food Sci. Biotechnol. 2013, 22, 1–4. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Tan, H.Y.; Chen, J.; Wang, M. Characterization of tyrosinase inhibitors in the twigs of Cudrania tricuspidata and their structure-activity relationship study. Fitoterapia 2013, 84, 242–247. [Google Scholar] [CrossRef]
- Chen, X.X.; Shi, Y.; Chai, W.M.; Feng, H.L.; Zhuang, J.X.; Chen, Q.X. Condensed tannins from Ficus virens as tyrosinase inhibitors: Structure, inhibitory activity and molecular mechanism. PLoS ONE 2014, 9, e91809. [Google Scholar] [CrossRef]
- Kim, Y.J.; Sohn, M.J.; Kim, W.G. Chalcomoracin and moracin C, new inhibitors of Staphylococcus aureus enoyl-acyl carrier protein reductase from Morus alba. Biol. Pharm. Bull. 2012, 35, 791–795. [Google Scholar] [CrossRef]
- Mannila, E.; Talvitie, A.; Kolehmainen, E. Anti-leukaemic compounds derived from stibenes in Picea abies bark. Phytochemistry 1993, 33, 813–816. [Google Scholar] [CrossRef]
- Thakkar, K.; Geahlen, R.L.; Cushman, M. Synthesis and protein-tyrosine kinase inhibitory activity of polyhydroxylated stilbene analogues of piceatannol. J. Med. Chem. 1993, 36, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Kuwahara, Y.; Suzuki, T. A New 2-arylbenzofuran, ω -hydroxy moracin N, from mulberry leaves. Agric. Biol. Chem. 1991, 55, 1409–1410. [Google Scholar] [CrossRef]
- Kuete, V.; Fozing, D.C.; Kapche, W.F.; Mbaveng, A.T.; Kuiate, J.R.; Ngadjui, B.T.; Abegaz, B.M. Antimicrobial activity of the methanolic extract and compounds from Morus mesozygia stem bark. J. Ethnopharmacol. 2009, 124, 551–555. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K. Stilbenes with tyrosinase inhibitory activity. Curr. Sci. 2008, 94, 44–52. [Google Scholar]
- Deguchi, T.; Tamai, A.; Asahara, K.; Miyamoto, K.; Miyamoto, A.; Nomura, M.; Kawata, T.T.; Yoshioka, Y.; Murata, K. Anti-tyrosinase and anti-oxidative activities by asana: The heartwood of Pterocarpus marsupium. Nat. Prod. Commun. 2019, 14, 1–9. [Google Scholar]
- Zheng, Z.P.; Cheng, K.W.; Zhu, Q.; Wang, X.C.; Lin, Z.X.; Wang, M. Tyrosinase inhibitory constituents from the roots of Morus nigra: A structure-activity relationship study. J. Agric. Food Chem. 2010, 58, 5368–5373. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, G.; Chen, J.; Zheng, Z.P. Characterization of a new flavone and tyrosinase inhibition constituents from the twigs of Morus alba L. Molecules 2016, 21, 1130. [Google Scholar] [CrossRef] [Green Version]
- Le, T.H.; Nguyen, H.X.; Do, T.V.N.; Dang, P.H.; Nguye, N.T.; Nguyena, M.T.T.N. Moracin VN, a new tyrosinase and xanthine oxidase inhibitor from the woods of Artocarpus heterophyllus. Nat. Prod. Commun. 2017, 12, 925–927. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Choi, S.W. Isolation, identification, and quantification of tyrosinase and alpha-glucosidase inhibitors from UVC-irradiated mulberry (Morus alba L.) leaves. Prev. Nutr. Food Sci. 2019, 24, 84–94. [Google Scholar] [CrossRef]
- Huang, Y.C.; Liu, K.C.; Chiou, Y.L. Melanogenesis of murine melanoma cells induced by hesperetin, a Citrus hydrolysate-derived flavonoid. Food Chem. Toxicol. 2012, 71, 653–659. [Google Scholar] [CrossRef]
- Ohguchi, K.; Ahao, Y.; Nozawa, Y. Stimulation of melanogenesis by the citrus flavonoid naringenin in mouse B16 melanoma cells. Biosci. Biotechnol. Biochem. 2006, 70, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, H.; Chu, J.H.; Chou, G.X.; Yu, Z.L. Activation of p38 MAPK pathway contributes to the melanogenic property of apigenin in B16 cells. Exp. Dermatol. 2011, 20, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Aneklaphakij, C.; Chamnanpuen, P.; Bunsupa, S.; Satitpatipan, V. Recent Green Technologies in Natural Stilbenoids Production and Extraction: The Next Chapter in the Cosmetic Industry. Cosmetics 2022, 9, 91. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.-M.; Zhang, J.; Zhang, Y.-Q. An efficient preparation of mulberroside A from the branch bark of mulberry and its effect on the inhibition of tyrosinase activity. PLoS ONE 2014, 9, e109396. [Google Scholar] [CrossRef]
- Lorian, V. Antibiotics in Laboratory Medicine, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Testes for Bacterial That Grow Aerobically: Approved Standards, 7th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2006. [Google Scholar]
- Sritularak, B.; De-Eknamkul, W.; Likhitwitayawuid, K. Tyrosinase inhibitors from Artocarpus lakoocha. Thai J. Pharm Sci. 1998, 22, 149–155. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Takahashi, H.; Parsons, P.G. Rapid and reversible inhibition of tyrosinase activity by glucosidase inhibitors in human melanoma cells. J. Investig. Dermatol. 1992, 98, 481–487. [Google Scholar] [CrossRef]
- Hunt, G.; Todd, C.; Cresswell, J.E.; Thody, A.J. Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affectmorphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J. Cell Sci. 1994, 107, 205–211. [Google Scholar] [CrossRef]
- Ye, Y.; Chou, G.X.; Mu, D.D.; Wang, H.; Chu, J.H.; Leung, A.K. Screening of Chinese herbal medicines for antityrosinase activity in a cell free system and B16 cells. J. Ethnopharmacol. 2010, 129, 387–390. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Phoopha, S.; Wattanapiromsakul, C.; Pitakbut, T.; Dej-Adisai, S. A New stilbene derivative and isolated compounds from Bauhinia Pottsii Var. Pottsii with their anti-alpha-glucosidase activity. Pharmacogn. Mag. 2020, 16, 161. [Google Scholar]
- Pitakbut, T.; Nguyen, G.-N.; Kayser, O. Activity of THC, CBD, and CBN on human ACE2 and SARS-CoV1/2 main protease to understand antiviral defense mechanism. Planta Med. 2022, 88, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Sakulkeo, O.; Wattanapiromsakul, C.; Pitakbut, T.; Dej-adisai, S. Alpha-glucosidase inhibition and molecular docking of isolated compounds from traditional Thai medicinal plant, Neuropeltis Racemosa Wall. Molecules 2022, 27, 639. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biovia Discovery Studio. Discovery Studio Visualizer, version v21.1.0.20298; Biovia Discovery Studio: San Diego, CA, USA, 2017. [Google Scholar]







| Compounds | S. epidermidis | S. aureus | MRSA | C. acnes | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (µg/mL) | MBC µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| 1 | 16 | 32 | 16 | 32 | 16 | >256 | 128 | >256 |
| 2 | 64 | >256 | 256 | >256 | 64 | >256 | >256 | >256 |
| 3 | 128 | >256 | 32 | 64 | 32 | 64 | 128 | >256 |
| 4 | 128 | >256 | 128 | 256 | 64 | 256 | >256 | >256 |
| 5 | 64 | 128 | >256 | >256 | 128 | >256 | >256 | >256 |
| Oxa P | 0.5 | 0.5 | 0.125 | 0.125 | NT | NT | 0.5 | 0.5 |
| Van P | NT | NT | NT | NT | 0.125 | 0.5 | NT | NT |
| Compound | Name | IC50 (µg/mL) |
|---|---|---|
| 1 | ω-hydroxymoracin C | 109.64 ± 0.89 |
| 2 | moracin M | 47.34 ± 0.78 |
| 3 | moracin C | 128.67 ± 1.21 |
| 4 | 3, 4, 3′, 5′-tetrahydroxybibenzyl | 35.65 ± 0.98 |
| 5 | piceatannol | 149.73 ± 0.86 |
| Std. | Kojic acid P | 38.67 ± 0.94 |
| Std. | Water extract of A. lacucha wood P | 8.73 ± 0.69 |
| Residues | Compound 1 | Compound 2 | Compound 3 | Compound 4 | Compound 5 | Conserve |
|---|---|---|---|---|---|---|
| ω-Hydroxymoracin C | Moracin M | Moracin C | 3, 4, 3´, 5´-Tetrahydroxybibenzyl | Piceatannol | ||
| His61 | ||||||
| His85 | ✓ | ✓ | MM | |||
| Phe90 | ||||||
| Phe192 | ||||||
| Val248 | ✓ | ✓ | SS | |||
| Met257 | ||||||
| His259 | ✓ | ✓ | MM | |||
| Asn260 | ||||||
| His263 | ✓ | ✓ | SS | |||
| Phe264 | ✓ | ✓ | ✓ | MMS | ||
| Arg268 | ||||||
| Pro277 | ✓ | ✓ | MM | |||
| His279 | ||||||
| Met280 | ✓ | |||||
| Gly281 | ||||||
| Ser282 | ✓ | ✓ | ✓ | MMM | ||
| Val283 | ✓ | ✓ | ✓ | ✓ | ✓ | XXXXX |
| Ala286 | ✓ | ✓ | SS |
| Autodock 4.2.6 | Experiment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmp | vdW+ Hbond (1) | Elec Stat. Energy (2) | Desol. Energy (3) | Total Intermol. Interact. Energy (4; 1 + 2 + 3) | Total Internal Energy (5) | Tors. Free Energy (6) | Unbound’s Energy (7) | Estimated Binding Energy (Kcal/mol) (8; 4 + 5 + 6 + 7) | Modified Estimated Binding Energy (Kcal/mol) (9; 4 + 5 + 7) | IC50 Values |
| 1 | −7.47 | 0.01 | 2.75 | −4.71 | −0.74 | 2.39 | 0.00 | −3.06 | −5.45 | 109.64 |
| 2 * | −6.09 | 0.08 | 2.29 | −3.73 | −0.08 | 1.19 | 0.00 | −2.62 * | −3.81 * | 47.34 |
| 3 | −6.83 | 0.02 | 2.08 | −4.73 | −0.37 | 1.79 | 0.00 | −3.31 | −5.10 | 128.67 |
| 4 | −8.70 | 0.04 | 3.21 | −5.45 | −0.34 | 2.09 | 0.00 | −3.70 | −5.79 | 38.67 |
| 5 | −8.08 | 0.07 | 3.06 | −4.94 | −0.36 | 1.79 | 0.00 | −3.51 | −5.30 | 149.73 |
| 0.46 | 0.89 | 1 | ||||||||
| Correlation to the IC50 value | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parndaeng, K.; Pitakbut, T.; Wattanapiromsakul, C.; Hwang, J.S.; Udomuksorn, W.; Dej-adisai, S. Chemical Constituents from Streblus taxoides Wood with Their Antibacterial and Antityrosinase Activities Plus in Silico Study. Antibiotics 2023, 12, 319. https://doi.org/10.3390/antibiotics12020319
Parndaeng K, Pitakbut T, Wattanapiromsakul C, Hwang JS, Udomuksorn W, Dej-adisai S. Chemical Constituents from Streblus taxoides Wood with Their Antibacterial and Antityrosinase Activities Plus in Silico Study. Antibiotics. 2023; 12(2):319. https://doi.org/10.3390/antibiotics12020319
Chicago/Turabian StyleParndaeng, Kedsaraporn, Thanet Pitakbut, Chatchai Wattanapiromsakul, Jae Sung Hwang, Wandee Udomuksorn, and Sukanya Dej-adisai. 2023. "Chemical Constituents from Streblus taxoides Wood with Their Antibacterial and Antityrosinase Activities Plus in Silico Study" Antibiotics 12, no. 2: 319. https://doi.org/10.3390/antibiotics12020319
APA StyleParndaeng, K., Pitakbut, T., Wattanapiromsakul, C., Hwang, J. S., Udomuksorn, W., & Dej-adisai, S. (2023). Chemical Constituents from Streblus taxoides Wood with Their Antibacterial and Antityrosinase Activities Plus in Silico Study. Antibiotics, 12(2), 319. https://doi.org/10.3390/antibiotics12020319