Antibiofilm Effect of Biogenic Silver Nanoparticles Combined with Oregano Derivatives against Carbapenem-Resistant Klebsiella pneumoniae
Abstract
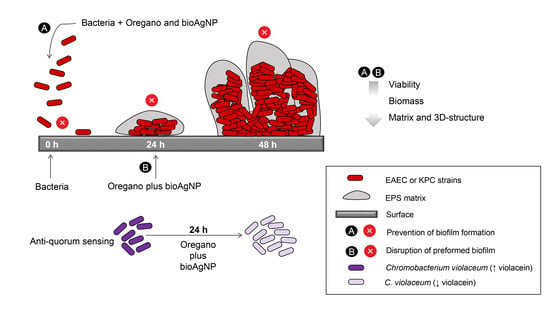
:1. Introduction
2. Results
2.1. Biogenic Silver Nanoparticles (bioAgNP) Characterization Biogenic Silver Nanoparticle
2.2. Sessile Minimal Inhibitory Concentration (SMIC) of Compounds Alone against Both Initial Stage of Biofilm Formation and Preformed Biofilm
2.3. Sessile Minimal Inhibitory Concentration (SMIC) of Compounds in Combinations against Both the Initial Stage of Biofilm Formation and Preformed Biofilm
2.4. Antibiofilm Effect of Binary Combinations Compared to Antimicrobials Individually against Preformed Biofilm in Microtiter Plates and Its Initial Stage of Formation (Reduction in Biomass and Metabolic Activity)
2.5. Scanning Electron Microscopy Study of Preformed Biofilm Treated with Compounds Alone and in Combination
2.6. Scanning Electron Microscopy Study of 24 h-Biofilms of EAEC and KPC Strains
2.7. Effect of Compounds on Violacein Production
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antibacterial Agents
4.2.1. Oregano-Derived Compounds
4.2.2. Biogenically Synthetized Silver Nanoparticles (bioAgNP)
4.3. Antibiofilm Assays
4.3.1. Biofilm Quantification by Chemical Methods (Crystal Violet and MTT)
4.3.2. Quorum Sensing Inhibition Test Based on C. violaceum
Determination of Subinhibitory Antibacterial Concentrations
Violacein Inhibition Assay
4.3.3. Scanning Electron Microscopy (SEM) Study of Antibiofilm Effect of Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Food and Drug Administration Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 21 February 2023).
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.H.; Esposito, E. Mechanistic Aspects of Biosynthesis of Silver Nanoparticles by Several Fusarium Oxysporum Strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- World Health Organization Food Safety. Available online: https://www.who.int/health-topics/food-safety#tab=tab_1 (accessed on 21 February 2023).
- Grigore-Gurgu, L.; Bucur, F.I.; Borda, D.; Alexa, E.-A.; Neagu, C.; Nicolau, A.I. Biofilms Formed by Pathogens in Food and Food Processing Environments. In Bacterial Biofilms; Springer Science & Business Media: Berlin, Germany, 2019. [Google Scholar]
- de Campos, A.C.L.P.; Nandi, R.D.S.; Scandorieiro, S.; Gonçalves, M.C.; Reis, G.F.; Dibo, M.; Medeiros, L.P.; Panagio, L.A.; Fagan, E.P.; Kobayashi, R.K.T.; et al. Antimicrobial Effect of Origanum Vulgare (L.) Essential Oil as an Alternative for Conventional Additives in the Minas Cheese Manufacture. LWT 2022, 157, 113063. [Google Scholar] [CrossRef]
- Debiagi, F.; Kobayashi, R.K.T.; Nakazato, G.; Panagio, L.A.; Mali, S. Biodegradable Active Packaging Based on Cassava Bagasse, Polyvinyl Alcohol and Essential Oils. Ind. Crops Prod. 2014, 52, 664–670. [Google Scholar] [CrossRef]
- das Neves, M.D.S.; Scandorieiro, S.; Pereira, G.N.; Ribeiro, J.M.; Seabra, A.B.; Dias, A.P.; Yamashita, F.; Martinez, C.B.D.R.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial Activity of Biodegradable Films Incorporated with Biologically-Synthesized Silver Nanoparticles and the Evaluation of Their Migration to Chicken Meat. Antibiotics 2023, 12, 178. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, R.; Mohammadzadeh, R.; Alikhani, M.Y.; Shokri Moghadam, M.; Karampoor, S.; Kazemi, S.; Barfipoursalar, A.; Yousefimashouf, R. The Biofilm-Associated Bacterial Infections Unrelated to Indwelling Devices. IUBMB Life 2020, 72, 1271–1285. [Google Scholar] [CrossRef]
- Abdullahi, U.F.; Igwenagu, E.; Mu’azu, A.; Aliyu, S.; Umar, M.I. Intrigues of Biofilm: A Perspective in Veterinary Medicine. Vet. World 2016, 9, 12–18. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, 181–190. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, Pathogenesis and Prevention—A Journey to Break the Wall: A Review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Gupta, S.; Laskar, N.; Kadouri, D.E. Evaluating the Effect of Oxygen Concentrations on Antibiotic Sensitivity, Growth, and Biofilm Formation of Human Pathogens. Microbiol. Insights 2016, 9, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of Bacterial Efflux Pumps in Biofilm Formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [Green Version]
- Balcázar, J.L.; Subirats, J.; Borrego, C.M. The Role of Biofilms as Environmental Reservoirs of Antibiotic Resistance. Front. Microbiol. 2015, 6, 1216. [Google Scholar] [CrossRef] [Green Version]
- Antimicrobial Resistance Collaborators Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [CrossRef]
- O’Neil, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust and HM Government: London, UK, 2016. [Google Scholar]
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 21 February 2023).
- Centers for Disease Control and Prevention Antibiotic Resistance: A Global Threat. Available online: https://www.cdc.gov/drugresistance/solutions-initiative/stories/ar-global-threat.html (accessed on 21 February 2023).
- World Health Organization Lack of New Antibiotics Threatens Global Efforts to Contain Drug-Resistant Infections. Available online: https://www.who.int/news/item/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections#:~:text=Lack%20of%20new%20antibiotics%20threatens%20global%20efforts%20to%20contain%20drug%2Dresistant%20infections,-17%20January%202020&text=Declining%20private%20investment%20and%20lack,World%20Health%20Organization%20(WHO) (accessed on 21 February 2023).
- Feldman, C.; Anderson, R. The Role of Co-Infections and Secondary Infections in Patients with COVID-19. Pneumonia 2021, 13, 5. [Google Scholar] [CrossRef]
- Al-Shammary, A.H.A.; Mounam, M.A.W.A. The Effect of Oak, Cinnamon, Oregano and Thyme Extracts on Biofilm Producing ESBL Klebsiella Pneumonia. Int. J. Pharma. Sci. 2017, 7, 1839–1847. [Google Scholar]
- Amaral, V.C.S.; Santos, P.R.; da Silva, A.F.; dos Santos, A.R.; Machinski, M.; Mikcha, J.M.G. Effect of Carvacrol and Thymol on Salmonella Spp. Biofilms on Polypropylene. Int. J. Food Sci. Technol. 2015, 50, 2639–2643. [Google Scholar] [CrossRef]
- García-Heredia, A.; García, S.; Merino-Mascorro, J.Á.; Feng, P.; Heredia, N. Natural Plant Products Inhibits Growth and Alters the Swarming Motility, Biofilm Formation, and Expression of Virulence Genes in Enteroaggregative and Enterohemorrhagic Escherichia coli. Food Microbiol. 2016, 59, 124–132. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-Biofilm Effect of Selected Essential Oils and Main Components on Mono- and Polymicrobic Bacterial Cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef] [Green Version]
- Raei, P.; Pourlak, T.; Memar, M.Y.; Alizadeh, N.; Aghamali, M.; Zeinalzadeh, E.; Asgharzadeh, M.; Kafil, H.S. Thymol and Carvacrol Strongly Inhibit Biofilm Formation and Growth of Carbapenemase-Producing Gram Negative Bacilli. Cell Mol. Biol. 2017, 63, 108–112. [Google Scholar] [CrossRef]
- Scandorieiro, S.; Rodrigues, B.C.D.; Nishio, E.K.; Panagio, L.A.; de Oliveira, A.G.; Durán, N.; Nakazato, G.; Kobayashi, R.K.T. Biogenic Silver Nanoparticles Strategically Combined with Origanum Vulgare Derivatives: Antibacterial Mechanism of Action and Effect on Multidrug-Resistant Strains. Front. Microbiol. 2022, 13, 1083. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; Coppola, R.; Jesus Ayala-Zavala, F.; Gomez da Cruz, A.; De Feo, V. Essential Oils and Microbial Communication. In Essential Oils; IntechOpen: London, UK, 2019. [Google Scholar]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Gutierrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Rodriguez-Garcia, I.; Gonzalez-Aguilar, G.A.; Ponce, A.; Moreira, M.d.R.; Roura, S.I.; Ayala-Zavala, J.F. Oregano Essential Oil-Pectin Edible Films as Anti-Quorum Sensing and Food Antimicrobial Agents. Front. Microbiol. 2014, 5, 699. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Nowack, B.; Krug, H.F.; Height, M. 120 Years of Nanosilver History: Implications for Policy Makers. Env. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef]
- Nakazato, G.; Kobayashi, R.K.T.; Seabra, A.B.; Duran, N. Use of Nanoparticles as a Potential Antimicrobial for Food Packaging. In Food Preservation; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 413–447. [Google Scholar]
- Nakazato, G.; Lonni, A.A.S.G.; Panagio, L.A.; de Camargo, L.C.; Gonçalves, M.C.; Reis, G.F.; Miranda-Sapla, M.M.; Tomiotto-Pellissier, F.; Kobayashi, R.K.T. Applications of Nanometals in Cutaneous Infections. In Nanotechnology in Skin, Soft Tissue, and Bone Infections; Rai, M., Ed.; Spriger: Berlin/Heidelberg, Germany, 2020; pp. 71–92. [Google Scholar]
- Kobayashi, R.K.T.; Nishio, E.K.; Scandorieiro, S.; Saikawa, G.I.A.; Da Rocha, S.P.D.; Nakazato, G. Metallic Nanoparticles as a Potential Antimicrobial for Catheters and Prostheses. In Materials for Biomedical Engineering; Grumezescu, A., Butu, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–196. [Google Scholar]
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.R.S.S.; Garnaes, J.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, R.V.; Lütken, H.; et al. Anti-Biofilm Effects of Gold and Silver Nanoparticles Synthesized by the Rhodiola Rosea Rhizome Extracts. Artif. Cells Nanomed. Biotechnol. 2018, 46, S886–S899. [Google Scholar] [CrossRef] [Green Version]
- Siddique, M.H.; Aslam, B.; Imran, M.; Ashraf, A.; Nadeem, H.; Hayat, S.; Khurshid, M.; Afzal, M.; Malik, I.R.; Shahzad, M.; et al. Effect of Silver Nanoparticles on Biofilm Formation and EPS Production of Multidrug-Resistant Klebsiella Pneumoniae. Biomed. Res. Int. 2020, 2020, 6398165. [Google Scholar] [CrossRef] [Green Version]
- Meza-Villezcas, A.; Gallego-Hernández, A.L.; Yildiz, F.H.; Jaime-Acuña, O.E.; Raymond-Herrera, O.; Huerta-Saquero, A. Effect of Antimicrobial Nanocomposites on Vibrio Cholerae Lifestyles: Pellicle Biofilm, Planktonic and Surface-Attached Biofilm. PLoS ONE 2019, 14, e0217869. [Google Scholar] [CrossRef]
- Andrade, P.F.; Nakazato, G.; Durán, N. Additive Interaction of Carbon Dots Extracted from Soluble Coffee and Biogenic Silver Nanoparticles against Bacteria. J. Phys. Conf. Ser. 2017, 838, 012028. [Google Scholar] [CrossRef]
- Graves, J.L.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid Evolution of Silver Nanoparticle Resistance in Escherichia coli. Front. Genet. 2015, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Muller, M. Bacterial Silver Resistance Gained by Cooperative Interspecies Redox Behavior. Antimicrob. Agents Chemother. 2018, 62, e00672-18. [Google Scholar] [CrossRef] [Green Version]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial Resistance to Silver Nanoparticles and How to Overcome It. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.C.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.T.J.; Duran, N.; Nakazato, G.; Kobayashi, R.K.T. Synergistic and Additive Effect of Oregano Essential Oil and Biological Silver Nanoparticles against Multidrug-Resistant Bacterial Strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef]
- Høiby, N. A Personal History of Research on Microbial Biofilms and Biofilm Infections. Pathog. Dis. 2014, 70, 205–211. [Google Scholar] [CrossRef] [Green Version]
- de Lima, R.; Seabra, A.B.; Durán, N. Silver Nanoparticles: A Brief Review of Cytotoxicity and Genotoxicity of Chemically and Biogenically Synthesized Nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef]
- Marcato, P.D.; Nakasato, G.; Brocchi, M.; Melo, P.S.; Huber, S.C.; Ferreira, I.R.; Alves, O.L.; Durán, N. Biogenic Silver Nanoparticles: Antibacterial and Cytotoxicity Applied to Textile Fabrics. J. Nano Res. 2012, 20, 69–76. [Google Scholar] [CrossRef]
- Haney, E.F.; Trimble, M.J.; Cheng, J.T.; Vallé, Q.; Hancock, R.E.W. Critical Assessment of Methods to Quantify Biofilm Growth and Evaluate Antibiofilm Activity of Host Defence Peptides. Biomolecules 2018, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Wang, C.; Chen, Z.; Allan, E.; van der Mei, H.C.; Busscher, H.J. Emergent Heterogeneous Microenvironments in Biofilms: Substratum Surface Heterogeneity and Bacterial Adhesion Force-Sensing. FEMS Microbiol. Rev. 2018, 42, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Hannig, C.; Follo, M.; Hellwig, E.; Al-Ahmad, A. Visualization of Adherent Micro-Organisms Using Different Techniques. J. Med Microbiol. 2010, 59, 1–7. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial Adhesion and Biofilms on Surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Williams, D.L.; Smith, S.R.; Peterson, B.R.; Allyn, G.; Cadenas, L.; Epperson, R.T.; Looper, R.E. Growth Substrate May Influence Biofilm Susceptibility to Antibiotics. PLoS ONE 2019, 14, e0206774. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, H.; Duck, Z.; Lilley, K.S.; Welch, M. Interrelationships between Colonies, Biofilms, and Planktonic Cells of Pseudomonas Aeruginosa. J. Bacteriol. 2007, 189, 2411–2416. [Google Scholar] [CrossRef] [Green Version]
- Wood, T.K. Insights on Escherichia coli Biofilm Formation and Inhibition from Whole-Transcriptome Profiling. Environ Microbiol 2009, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.M.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E.W. Inhibition of Bacterial Biofilm Formation and Swarming Motility by a Small Synthetic Cationic Peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [Green Version]
- Saleem, S.; Ahmed, B.; Khan, M.S.; Al-Shaeri, M.; Musarrat, J. Inhibition of Growth and Biofilm Formation of Clinical Bacterial Isolates by NiO Nanoparticles Synthesized from Eucalyptus Globulus Plants. Microb. Pathog. 2017, 111, 375–387. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yun, W.; Lee, J.H.; Lee, C.H.; Kwak, W.K.; Cho, J.H. Effects of Essential Oil (Blended and Single Essential Oils) on Anti-Biofilm Formation of Salmonella and Escherichia coli. J. Anim. Sci. Technol. 2017, 59, 4. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, Y.G.; Lee, J. Carvacrol-Rich Oregano Oil and Thymol-Rich Thyme Red Oil Inhibit Biofilm Formation and the Virulence of Uropathogenic Escherichia coli. J. Appl. Microbiol. 2017, 123, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Pal, R. Antibiofilm Efficacy of Silver Nanoparticles against Biofilm of Extended Spectrum β-Lactamase Isolates of Escherichia coli and Klebsiella Pneumoniae. Appl. Nanosci. 2014, 4, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli Biofilm: Development and Therapeutic Strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Diggikar, R.S.; Patil, R.H.; Kale, S.B.; Thombre, D.K.; Gade, W.N.; Kulkarni, M.V.; Kale, B.B. Silver-Decorated Orthorhombic Nanotubes of Lithium Vanadium Oxide: An Impeder of Bacterial Growth and Biofilm. Appl. Microbiol. Biotechnol. 2013, 97, 8283–8290. [Google Scholar] [CrossRef]
- Shafreen, R.B.; Seema, S.; Ahamed, A.P.; Thajuddin, N.; Ali Alharbi, S. Inhibitory Effect of Biosynthesized Silver Nanoparticles from Extract of Nitzschia Palea Against Curli-Mediated Biofilm of Escherichia coli. Appl. Biochem. Biotechnol. 2017, 183, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, S.; Gondil, V.S.; Sharma, S.; Kumar, M.; Wangoo, N.; Sharma, R.K. A Novel Approach for Combating Klebsiella Pneumoniae Biofilm Using Histidine Functionalized Silver Nanoparticles. Front. Microbiol. 2017, 8, 1104. [Google Scholar] [CrossRef] [Green Version]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Durán, N.; Nakazato, G.; Seabra, A.B. Antimicrobial Activity of Biogenic Silver Nanoparticles, and Silver Chloride Nanoparticles: An Overview and Comments. Appl. Microbiol. Biotechnol. 2016, 100, 6555–6570. [Google Scholar] [CrossRef]
- Nisar, P.; Ali, N.; Rahman, L.; Ali, M.; Shinwari, Z.K. Antimicrobial Activities of Biologically Synthesized Metal Nanoparticles: An Insight into the Mechanism of Action. J. Biol. Inorg. Chem. 2019, 24, 929–941. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, J.; Hicks, S.; Dall’agnol, M.; Phillips, A.D.; Nataro, J.P. Roles for Fis and YafK in Biofilm Formation by Enteroaggregative Escherichia coli. Mol. Microbiol. 2001, 41, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanjee, U.; Houry, W.A. Mechanisms of Acid Resistance in Escherichia coli. Annu. Rev. Microbiol. 2013, 67, 65–81. [Google Scholar] [CrossRef] [Green Version]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus Cereus. Appl. Env. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Yadav, S.; Chauhan, B.S.; Nandy, N.; Singh, R.; Neogi, K.; Roy, J.K.; Srikrishna, S.; Singh, R.K.; Prakash, P. Classification of Clinical Isolates of Klebsiella Pneumoniae Based on Their in Vitro Biofilm Forming Capabilities and Elucidation of the Biofilm Matrix Chemistry with Special Reference to the Protein Content. Front. Microbiol. 2019, 10, 669. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.; Bradshaw, S.B. Biofilm Formation by the Enterobacteriaceae: A Comparison between Salmonella Enteritidis, Escherichia coli and a Nitrogen-Fixing Strain of Klebsiella Pneumoniae. J. Appl. Bacteriol. 1996, 80, 458–464. [Google Scholar] [CrossRef]
- Baugh, S.; Phillips, C.R.; Ekanayaka, A.S.; Piddock, L.J.V.; Webber, M.A. Inhibition of Multidrug Efflux as a Strategy to Prevent Biofilm Formation. J. Antimicrob. Chemother. 2014, 69, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The Natural Antimicrobial Carvacrol Inhibits Quorum Sensing in Chromobacterium Violaceum and Reduces Bacterial Biofilm Formation at Sub-Lethal Concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.G.; Ansari, M.A.; Sajid Jamal, Q.M.; Khan, H.M.; Jalal, M.; Ahmad, H.; Mahdi, A.A. Antiquorum Sensing Activity of Silver Nanoparticles in P. Aeruginosa: An in Silico Study. Silico Pharm. 2017, 5, 12. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as Potential Quorum Sensing Inhibitor of Pseudomonas Aeruginosa and Biofilm Production on Stainless Steel Surfaces. Food Control. 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, W.; Yuk, H.G. Effects of Sublethal Thymol, Carvacrol, and Trans-Cinnamaldehyde Adaptation on Virulence Properties of Escherichia coli O157: H7. Appl. Env. Microbiol. 2019, 85, e00271-19. [Google Scholar] [CrossRef] [Green Version]
- Reichling, J. Anti-Biofilm and Virulence Factor-Reducing Activities of Essential Oils and Oil Components as a Possible Option for Bacterial Infection Control. Planta Med. 2020, 86, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Johnjulio, W.; Fuge, L.H.; Kad, M.; Post, C. Introduction to Biofilms in Family Medicine. South Med. J. 2012, 105, 24–29. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A Phyto-Compound Effective against Methicillin-Resistant and Methicillin-Sensitive Staphylococcus Aureus Clinical Strain Biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef] [Green Version]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of Oregano, Carvacrol and Thymol on Staphylococcus Aureus and Staphylococcus Epidermidis Biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial Activity of Naturally Occurring Phenols and Derivatives Against Biofilm and Planktonic Bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Radzig, M.A.; Nadtochenko, V.A.; Koksharova, O.A.; Kiwi, J.; Lipasova, V.A.; Khmel, I.A. Antibacterial Effects of Silver Nanoparticles on Gram-Negative Bacteria: Influence on the Growth and Biofilms Formation, Mechanisms of Action. Colloids Surf. B Biointerfaces 2013, 102, 300–306. [Google Scholar] [CrossRef]
- Pompilio, A.; Geminiani, C.; Bosco, D.; Rana, R.; Aceto, A.; Bucciarelli, T.; Scotti, L.; Di Bonaventura, G. Electrochemically Synthesized Silver Nanoparticles Are Active against Planktonic and Biofilm Cells of Pseudomonas Aeruginosa and Other Cystic Fibrosis-Associated Bacterial Pathogens. Front. Microbiol. 2018, 9, 1349. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Qin, S.; Wei, Y.; Liu, S.; Peng, H.; Li, Q.; Luo, L.; Lv, M. Silver Nanoparticles Exert Concentration-Dependent Influences on Biofilm Development and Architecture. Cell Prolif. 2019, 52, e12616. [Google Scholar] [CrossRef]
- Ikuma, K.; Decho, A.W.; Lau, B.L.T. When Nanoparticles Meet Biofilms-Interactions Guiding the Environmental Fate and Accumulation of Nanoparticles. Front. Microbiol. 2015, 6, 591. [Google Scholar] [CrossRef]
- Kotronia, M.; Kavetsou, E.; Loupassaki, S.; Kikionis, S.; Vouyiouka, S.; Detsi, A. Encapsulation of Oregano (Origanum Onites l.) Essential Oil in β-Cyclodextrin (β-CD): Synthesis and Characterization of the Inclusion Complexes. Bioengineering 2017, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Losasso, C.; Belluco, S.; Cibin, V.; Zavagnin, P.; Mičetić, I.; Gallocchio, F.; Zanella, M.; Bregoli, L.; Biancotto, G.; Ricci, A. Antibacterial Activity of Silver Nanoparticles: Sensitivity of Different Salmonella Serovars. Front. Microbiol. 2014, 5, 227. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Horinouchi, T.; Furusawa, C. Acceleration and Suppression of Resistance Development by Antibiotic Combinations. BMC Genom. 2017, 18, 328. [Google Scholar] [CrossRef] [Green Version]
- Tyers, M.; Wright, G.D. Drug Combinations: A Strategy to Extend the Life of Antibiotics in the 21st Century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Cardozo, V.F.; Oliveira, A.G.; Nishio, E.K.; Perugini, M.R.E.; Andrade, C.G.T.J.; Silveira, W.D.; Durán, N.; Andrade, G.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial Activity of Extracellular Compounds Produced by a Pseudomonas Strain against Methicillin-Resistant Staphylococcus Aureus (MRSA) Strains. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Kora, A.J.; Rastogi, L. Enhancement of Antibacterial Activity of Capped Silver Nanoparticles in Combination with Antibiotics, on Model Gram-Negative and Gram-Positive Bacteria. Bioinorg. Chem. Appl. 2013, 2013, 871097. [Google Scholar] [CrossRef] [Green Version]
- Otaguiri, E.; Morguette, A.; Biasi-Garbin, R.; Morey, A.; Lancheros, C.; Kian, D.; Oliveira, A.; Kerbauy, G.; Perugini, M.; Duran, N.; et al. Antibacterial Combination of Oleoresin from Copaifera Multijuga Hayne and Biogenic Silver Nanoparticles Towards Streptococcus Agalactiae. Curr. Pharm. Biotechnol. 2016, 18, 177–190. [Google Scholar] [CrossRef]
- Longhi, C.; Santos, J.P.; Morey, A.T.; Marcato, P.D.; Duran, N.; Pinge-Filho, P.; Nakazato, G.; Yamada-Ogatta, S.F.; Yamauchi, L.M. Combination of Fluconazole with Silver Nanoparticles Produced by Fusarium Oxysporum Improves Antifungal Effect against Planktonic Cells and Biofilm of Drug-Resistant Candida Albicans. Med. Mycol. 2016, 54, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Bankier, C.; Matharu, R.K.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 16074. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of Antibiotics Antimicrobial Activity Due to the Silver Nanoparticles Impact on the Cell Membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Oliveira Junior, A.G.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; de Almeida, R.S.; Panagio, L.A. Antifungal Activity of Silver Nanoparticles and Simvastatin against Toxigenic Species of Aspergillus. Int. J. Food Microbiol. 2019, 291, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, N.H.; Tajik, H.; Moradi, M.; Kousheh, S.A.; Molaei, R. Antibacterial Interactions of Colloid Nanosilver with Eugenol and Food Ingredients. J. Food Prot. 2019, 82, 1783–1792. [Google Scholar] [CrossRef]
- Meroni, G.; Filipe, J.F.S.; Martino, P.A. In Vitro Antibacterial Activity of Biological-Derived Silver Nanoparticles: Preliminary Data. Vet. Sci. 2020, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Hamoud, R.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic Interactions in Two-Drug and Three-Drug Combinations (Thymol, EDTA and Vancomycin) against Multi Drug Resistant Bacteria Including E. Coli. Phytomedicine 2014, 21, 443–447. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial Activity of Essential Oils and Carvacrol, and Synergy of Carvacrol and Erythromycin, against Clinical, Erythromycin-Resistant Group A Streptococci. Front. Microbiol. 2015, 6, 164. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Synergistic Activities of Gaseous Oregano and Thyme Thymol Essential Oils against Listeria Monocytogenes on Surfaces of a Laboratory Medium and Radish Sprouts. Food Microbiol. 2020, 86, 103357. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y. Ben Antibacterial Activity and Mechanism of Silver Nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.H.; Mohamed, M.S.M.; Khalil, M.S.; Azmy, M.; Mabrouk, M.I. Combination of Essential Oil and Ciprofloxacin to Inhibit/Eradicate Biofilms in Multidrug-Resistant Klebsiella Pneumoniae. J. Appl. Microbiol. 2018, 125, 84–95. [Google Scholar] [CrossRef]
- Marslin, G.; Selvakesavan, R.K.; Franklin, G.; Sarmento, B.; Dias, A.C.P. Antimicrobial Activity of Cream Incorporated with Silver Nanoparticles Biosynthesized from Withania Somnifera. Int. J. Nanomed. 2015, 10, 5955–5963. [Google Scholar] [CrossRef] [Green Version]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakimoto, N.; Nishi, J.; Sheikh, J.; Nataro, J.P.; Sarantuya, J.; Iwashita, M.; Manago, K.; Tokuda, K.; Yoshinaga, M.; Kawano, Y. Quantitative biofilm assay using a microtiter plate to screen for enteroaggregative Escherichia coli. Am. J. Trop. Med. Hyg. 2004, 71, 687–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cady, N.C.; McKean, K.A.; Behnke, J.; Kubec, R.; Mosier, A.P.; Kasper, S.H.; Burz, D.S.; Musah, R.A. Inhibition of Biofilm Formation, Quorum Sensing and Infection in Pseudomonas Aeruginosa by Natural Products-Inspired Organosulfur Compounds. PLoS ONE 2012, 7, e38492. [Google Scholar] [CrossRef] [Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Traub, W.H.; Kleber, I. In Vitro Additive Effect of 0Polymyxin B and Rifampin Against Serratia Marcescens. Antimicrob. Agents Chemother. 1975, 7, 874–876. [Google Scholar] [CrossRef] [Green Version]
- National Committee for Clinical Laboratory Standards. Methods for Determining Bactericidal Activity of Antimicrobial Agents; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999. [Google Scholar]
- Blosser, R.S.; Gray, K.M. Extraction of Violacein from Chromobacterium Violaceum Provides a New Quantitative Bioassay for N-Acyl Homoserine Lactone Autoinducers. J. Microbiol. Methods 2000, 40, 47–55. [Google Scholar] [CrossRef]
- Erlandsen, S.L.; Kristich, C.J.; Dunny, G.M.; Wells, C.L. High-Resolution Visualization of the Microbial Glycocalyx with Low-Voltage Scanning Electron Microscopy: Dependence on Cationic Dyes. J. Histochem. Cytochem. 2004, 52, 1427–1435. [Google Scholar] [CrossRef] [Green Version]






| Antimicrobial | Formation | Preformed | Formation | Preformed |
|---|---|---|---|---|
| OEO | 0.30 | 0.59 | 0.59 | 0.59 |
| Car | 0.31 | 0.61 | 0.31 | 0.31 |
| Thy | 0.25 | 0.5 | 0.25 | 0.25 |
| bioAgNP | 0.98 | 31.5 | 1.97 | 7.88 |
| Antibacterial Binary Combinations | EAEC | KPC | ||||||
|---|---|---|---|---|---|---|---|---|
| Formation | Preformed | Formation | Preformed | |||||
| SMIC Combination | Fold Decrease | SMIC Combination | Fold Decrease | SMIC Combination | Fold Decrease | SMIC Combination | Fold Decrease | |
| OEO + bioAgNP | ||||||||
| OEO | >0.15 | no | 0.30 | 2× | 0.30 | 2× | >0.30 | no |
| bioAgNP | >0.25 | NT | 7.88 | 4× | 0.98 | 2× | >1.97 | NT |
| Car + bioAgNP | ||||||||
| Car | >0.15 | no | 0.31 | 2× | 0.15 | 2× | >0.15 | no |
| bioAgNP | >0.25 | NT | 7.88 | 4× | 0.98 | 2× | >1.97 | NT |
| Thy + bioAgNP | ||||||||
| Thy | 0.12 | 2× | 0.12 | 4× | 0.12 | 2× | >0.06 | NT |
| bioAgNP | 0.25 | 4× | 3.94 | 8× | 0.98 | 2× | >1.97 | NT |
| Car + Thy | ||||||||
| Car | 0.15 | 2× | >0.31 | no | >0.15 | no | >0.15 | no |
| Thy | 0.12 | 2× | >0.25 | no | >0.12 | no | >0.06 | NT |
| Bacteria | Antimicrobial Concentrations in Binary Combinations | Biofilm Reduction Caused by Combinations | Antibiofilm Effect of Combinations | Antimicrobial Concentrations Individually | Biofilm Reduction Caused by Antibacterials Alone | ||
|---|---|---|---|---|---|---|---|
| Biomass Decrease (%) | Metabolic Activity Decrease (%) | Biomass Decrease (%) | Metabolic Activity Decrease (%) | ||||
| EAEC | OEO + bioAgNP | Improved | OEO at 0.15mg/mL | 40 ± 1.9 | 3 ± 2.8 | ||
| 0.15mg/mL + 0.25 µg/mL | 88 ± 0.5 * | 70 ± 2.9 * | OEO at 0.07mg/mL | 19 ± 0.9 | 0 ± 0.0 | ||
| 0.07 mg/mL + 0.12 µg/mL | 49 ± 2.6 * | 6 ± 0.3 | Car at 0.15 mg/mL | 60 ± 2.8 | 2 ± 2.1 | ||
| Car + bioAgNP | Improved | Car at 0.08 mg/mL | 14± 1.8 | 0 ± 0.0 | |||
| 0.15 mg/mL + 0.25 µg/mL | 84 ± 0.7 * | 64 ± 1.5 * | Thy at 0.12 mg/mL | 0 ± 0.0 | 0 ± 0.0 | ||
| 0.08 mg/mL + 0.12 µg/mL | 51 ± 1.5 * | 9 ± 0.7 | Thy at 0.06 mg/mL | 0 ± 0.0 | 0 ± 0.0 | ||
| Thy + bioAgNP | Improved | bioAgNP at 0.25 µg/mL | 66 ± 1.2 | 27 ± 1.2 | |||
| 0.12 mg/mL + 0.25 µg/mL | 99 ± 0.6 * | 98 ± 0.1 * | bioAgNP at 0.12 µg/mL | 21 ± 1.4 | 13 ± 0.8 | ||
| 0.06 mg/mL + 0.12 µg/mL | 22 ± 1.4 | 9 ± 0.2 | |||||
| Car + Thy | Improved | ||||||
| 0.15 mg/mL + 0.12 mg/mL | 99 ± 0.5 * | 99 ± 0.1 * | |||||
| 0.08 mg/mL + 0.06 mg/mL | 62 ± 0.9 * | 19 ± 1.3 * | |||||
| Bacteria | Antimicrobial Concentrations in Binary Combinations | Biofilm Reduction Caused by Combinations | Antibiofilm Effect of Combinations | Antimicrobial Concentrations Individually | Biofilm Reduction Caused by Antibacterials Alone | ||
|---|---|---|---|---|---|---|---|
| Biomass Decrease (%) | Metabolic Activity Decrease (%) | Biomass Decrease (%) | Metabolic Activity Decrease (%) | ||||
| KPC | OEO + bioAgNP | Improved | OEO at 0.30mg/mL | 54 ± 1.7 | 42 ± 1.1 | ||
| 0.30 mg/mL+ 0.98 µg/mL | 97 ± 0.8 * | 99 ± 0.1 * | OEO at 0.15 mg/mL | 43 ± 1.9 | 33 ± 0.3 | ||
| 0.15 mg/mL + 0.49µg/mL | 54 ± 0.9 | 58 ± 2.1 * | Car at 0.15 µg/mL | 23 ± 0.3 | 24 ± 2.5 | ||
| Car + bioAgNP | Improved | Car at 0.08 µg/mL | 15 ± 1.9 | 27 ± 1.8 | |||
| 0.15 mg/mL + 0.98µg/mL | 97 ± 0.1 * | 99 ± 0.2 * | Thy at 0.12 µg/mL | 20 ± 3.1 | 6 ± 0.1 | ||
| 0.08 mg/mL + 049 µg/mL | 59 ± 1.7 * | 21 ± 2.6 | Thy at 0.06 µg/mL | 2 ± 0.7 | 0 ± 0.0 | ||
| Thy + bioAgNP | Improved | bioAgNP at 0.98 µg/mL | 0 ± 0.00 | 0 ± 0.0 | |||
| 0.12 mg/mL + 0.98 µg/mL | 99 ± 0.4 * | 100 ± 0.0 * | bioAgNP at 0.49 µg/mL | 0 ± 0.00 | 0 ± 0.0 | ||
| 0.06 mg/mL + 0.49 µg/mL | 12 ± 0.5 | 0 ± 0.0 | |||||
| Car + Thy | Similar | ||||||
| 0.15 mg/mL + 0.12 mg/mL | 34 ± 1.3 | 34 ± 2.9 | |||||
| 0.08 mg/mL + 0.06 mg/mL | 19 ± 1.6 | 8 ± 1.7 | |||||
| Bacteria | Antimicrobial Concentrations in Binary Combinations | Biofilm Reduction Caused by Combinations | Antibiofilm Effect of Combinations Compared to Antimicrobials Alone | Antimicrobial Concentrations Individually | Biofilm Reduction Caused by Antibacterials Alone |
|---|---|---|---|---|---|
| Metabolic Activity Decrease (%) | Metabolic Activity Decrease (%) | ||||
| EAEC | OEO + bioAgNP | Improved | OEO at 0.30 mg/mL | 19 ± 0.3 | |
| 0.30 mg/mL + 7.88 µg/mL | 99 ± 0.1 * | OEO at 0.15 mg/mL | 5 ± 0.9 | ||
| 0.15 mg/mL + 3.94 µg/mL | 41 ± 2.1 * | Car at 0.31 mg/mL | 12 ± 3.1 | ||
| Car + bioAgNP | Improved | Car at 0.15 mg/mL | 7 ± 0.6 | ||
| 0.31 mg/mL + 7.88 µg/mL | 99 ± 0.1 * | Thy at 0.25 mg/mL | 13 ± 1.2 | ||
| 0.15 mg/mL + 3.94 µg/mL | 26 ± 0.2 | Thy at 0.12 mg/mL | 1 ± 0.9 | ||
| Thy + bioAgNP | Improved | bioAgNP at 7.88 µg/mL | 70 ± 2.8 | ||
| 0.25 mg/mL + 7.88 µg/mL | 100 ± 0.0 * | bioAgNP at 3.94 µg/mL | 28 ± 1.8 | ||
| 0.12 mg/mL + 3.94 µg/mL | 98 ± 0.5 * | ||||
| Car + Thy | Improved | ||||
| 0.31 mg/mL + 0.25 mg/mL | 93 ± 0.2 * | ||||
| 0.15 mg/mL + 0.12 mg/mL | 92 ± 0.3 * |
| Bacteria | Antimicrobial Concentrations in Binary Combinations | Biofilm Reduction Caused by Combinations | Antibiofilm Effect of Combinations Compared to Antimicrobials Alone | Antimicrobial Concentrations Individually | Biofilm Reduction Caused by Antibacterials Alone |
|---|---|---|---|---|---|
| Metabolic Activity Decrease (%) | Metabolic Activity Decrease (%) | ||||
| KPC | OEO + bioAgNP | Improved | OEO at 0.30 mg/mL | 39 ± 0.1 | |
| 0.30 mg/mL + 1.97 µg/mL | 80 ± 1.1 * | OEO at 0.15 mg/mL | 31 ± 1.1 | ||
| 0.15 mg/mL + 0.98 µg/mL | 46 ± 1.6 * | Car at 0.15 mg/mL | 33 ± 0.8 | ||
| Car + bioAgNP | Similar | Car at 0.07 mg/mL | 34 ± 0.6 | ||
| 0.15 mg/mL + 1.97 µg/mL | 66 ± 0.9 | Thy at 0.06 mg/mL | 15 ± 0.6 | ||
| 0.07 mg/mL + 0.98 µg/mL | 37 ± 1.1 | Thy at 0.03 mg/mL | 10 ± 1.4 | ||
| Thy + bioAgNP | Similar | bioAgNP at 1.97 µg/mL | 55 ± 2.4 | ||
| 0.06 mg/mL + 1.97 µg/mL | 62 ± 3.2 | bioAgNP at 0.98 µg/mL | 0 ± 0.0 | ||
| 0.03 mg/mL + 0.98 µg/mL | 3 ± 0.9 | ||||
| Car + Thy | Improved | ||||
| 0.15 mg/mL + 0.06 mg/mL | 74 ± 1.4 * | ||||
| 0.08 mg/mL + 0.03 mg/mL | 53 ± 1.6 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scandorieiro, S.; Teixeira, F.M.M.B.; Nogueira, M.C.L.; Panagio, L.A.; de Oliveira, A.G.; Durán, N.; Nakazato, G.; Kobayashi, R.K.T. Antibiofilm Effect of Biogenic Silver Nanoparticles Combined with Oregano Derivatives against Carbapenem-Resistant Klebsiella pneumoniae. Antibiotics 2023, 12, 756. https://doi.org/10.3390/antibiotics12040756
Scandorieiro S, Teixeira FMMB, Nogueira MCL, Panagio LA, de Oliveira AG, Durán N, Nakazato G, Kobayashi RKT. Antibiofilm Effect of Biogenic Silver Nanoparticles Combined with Oregano Derivatives against Carbapenem-Resistant Klebsiella pneumoniae. Antibiotics. 2023; 12(4):756. https://doi.org/10.3390/antibiotics12040756
Chicago/Turabian StyleScandorieiro, Sara, Franciele Maira M. B. Teixeira, Mara C. L. Nogueira, Luciano A. Panagio, Admilton G. de Oliveira, Nelson Durán, Gerson Nakazato, and Renata K. T. Kobayashi. 2023. "Antibiofilm Effect of Biogenic Silver Nanoparticles Combined with Oregano Derivatives against Carbapenem-Resistant Klebsiella pneumoniae" Antibiotics 12, no. 4: 756. https://doi.org/10.3390/antibiotics12040756
APA StyleScandorieiro, S., Teixeira, F. M. M. B., Nogueira, M. C. L., Panagio, L. A., de Oliveira, A. G., Durán, N., Nakazato, G., & Kobayashi, R. K. T. (2023). Antibiofilm Effect of Biogenic Silver Nanoparticles Combined with Oregano Derivatives against Carbapenem-Resistant Klebsiella pneumoniae. Antibiotics, 12(4), 756. https://doi.org/10.3390/antibiotics12040756