Integration of Antimicrobials and Delivery Systems: Synergistic Antibiofilm Activity with Biodegradable Nanoemulsions Incorporating Pseudopyronine Analogs
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Activity of Analog Compounds against Gram-Positive Planktonic Bacteria
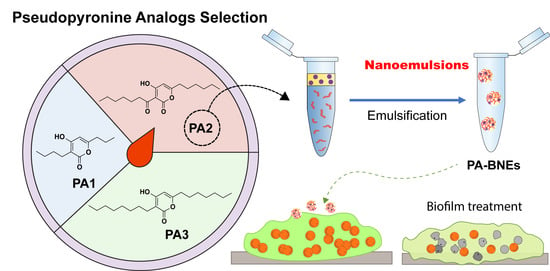
2.2. Fabrication of PAs-Loaded Biodegradable Nanoemulsions (PA-BNEs)
2.2.1. Solubility of PAs in Eugenol
2.2.2. Generation and Characterization of PA-BNEs
2.3. Antimicrobial Activity of PA-BNE
2.3.1. Antimicrobial Activity of PA-BNEs
2.3.2. Antibiofilm Activity of PA-BNEs
2.4. Cytotoxicity of PA-BNE In Vitro Fibroblast Cell
3. Discussion
4. Materials and Methods
4.1. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentrations (MBCs)
4.2. Preparation of PA-BNE
4.3. Minimal Biofilm Bactericidal Concentration (MBBC) Determination
4.4. Antibiofilm Study
4.5. Mammalian Cell Viability Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Antibiotic Resistance Threats in The United States 2019; U.S. Department of Human Health and Services, CDC: Atlanta, GA, USA, 2019; p. 10.
- Richardson, L.A. Understanding and Overcoming Antibiotic Resistance. PLoS Biol. 2017, 15, e2003775. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, E.B.; Tam, V.H. Impact of Multidrug-Resistant Pseudomonas aeruginosa Infection on Patient Outcomes. Expert. Rev. Pharmacoecon Outcomes Res. 2010, 10, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [Green Version]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Suzuki, A.; Fukuda, T.; Kobayashi, K.; Ohshiro, T.; Tomoda, H. Pseudopyronine B, an Inhibitor of Sterol O-acyltransferase, Produced by Pseudomonas sp. BYK11209. J. Antibiot. 2017, 70, 96–97. [Google Scholar] [CrossRef]
- McGlacken, G.P.; Fairlamb, I.J. 2-Pyrone Natural Products and Mimetics: Isolation, Characterization, and Biological Activity. Nat. Prod. Rep. 2005, 22, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Saurav, K.; Yu, Z.; Mándi, A.; Kurtán, T.; Li, J.; Tian, X.; Zhang, Q.; Zhang, W.; Zhang, C. α-Pyrones with Diverse Hydroxy Substitutions from Three Marine-Derived Nocardiopsis Strains. J. Nat. Prod. 2016, 79, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- McMullin, D.R.; Nsiama, T.K.; Miller, J.D. Isochromans and α-Pyrones from Penicillium corylophilum. J. Nat. Prod. 2014, 77, 206–212. [Google Scholar] [CrossRef]
- Lee, J.; Han, C.; Lee, T.G.; Chin, J.; Choi, H.; Lee, W.; Paik, M.J.; Won, D.H.; Jeong, G.; Ko, J.; et al. Marinopyrones A–D, α-Pyrones from Marine- Derived Actinomycetes of the Family Nocardiopsaceae. Tet. Lett. 2016, 57, 1997–2000. [Google Scholar] [CrossRef]
- Grundmann, F.; Dill, V.; Dowling, A.; Thanwisai, A.; Bode, E.; Chantratita, N.; Bode, H.B. Identification and Isolation of Insecticidal Oxazoles from Pseudomonas spp. Beilstein J. Org. Chem. 2012, 8, 749–752. [Google Scholar] [CrossRef] [Green Version]
- Chu, M.; Mierswa, R.; Xu, L.; He, J.; Terracciano, J.; Patel, M.; Zhao, W.; Black, T.A.; Chan, T.M. Structure of Sch 419560, a Novel α-Pyrone Antibiotic Produced by Pseudomonas fluorescens. J. Antibiot. 2002, 55, 215–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brachmann, A.O.; Brameyer, S.; Kresovic, D.; Hitkova, I.; Kopp, Y.; Manske, C.; Schubert, K.; Bode, H.B. Pyrones as Bacterial Signaling Molecules. Nat. Chem. Biol. 2013, 9, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Kong, F.; Janso, J.E.; Arias, D.A.; Suarez, P.A.; Bernan, V.S.; Petersen, P.J.; Weiss, W.J.; Carter, G.; Greenstein, M. Novel α-Pyrones Produced by a Marine Pseudomonas sp. F92S91: Taxonomy and Biological Activities. J. Antibiot. 2003, 56, 1033–1044. [Google Scholar]
- Bauer, S.J.; Ghequire, M.G.K.; Nett, M.; Josten, M.; Sahl, H.-G.; De Mot, R.; Gross, H. Biosynthetic Origin of the Antibiotic Pseudopyronines A and B in Pseudomonas putida BW11M1. ChemBioChem 2015, 16, 2491–2497. [Google Scholar] [CrossRef]
- Giddens, A.C.; Nielsen, L.; Boshoff, H.I.; Tasdemir, D.; Perozzo, R.; Kaiser, M.; Wang, F.; Sacchettini, J.C.; Copp, B.R. Natural Product Inhibitors of Fatty Acid Biosynthesis: Synthesis of the Marine Microbial Metabolites Pseudopyronines A and B and Evaluation of Their Anti-infective Activities. Tetrahedron 2008, 64, 1242–1249. [Google Scholar] [CrossRef]
- Bouthillette, L.M.; Darcey, C.A.; Handy, T.E.; Seaton, S.C.; Wolfe, A.L. Isolation of the Antibiotic Pseudopyronine B and SAR Evaluation of C3/C6 Alkyl Analogs. Bioorg. Med. Chem. Lett. 2017, 27, 2762–2765. [Google Scholar] [CrossRef]
- Ishikawa, M.; Hashimoto, Y. Improvement in Aqueous Solubility in Small Molecule Drug Discovery Programs by Disruption of Molecular Planarity and Symmetry. J. Med. Chem. 2011, 54, 1539–1554. [Google Scholar] [CrossRef] [PubMed]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of Intervention, Treatment, and Antibiotic Resistance of Biofilm-Forming Microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, M.; Bennett, R.N.; Rosa, E.A.S. Understanding Antimicrobial Activities of Phytochemicals against Multidrug-Resistant Bacteria and Biofilms. Nat. Prod. Rep. 2009, 26, 746. [Google Scholar] [CrossRef]
- Li, C.-H.; Chen, X.; Landis, R.F.; Geng, Y.; Makabenta, J.M.; Lemnios, W.; Gupta, A.; Rotello, V.M. Phytochemical-Based Nanocomposites for the Treatment of Bacterial Biofilms. ACS Infect. Dis. 2019, 5, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Makabenta, J.M.V.; Schlüter, F.; Landis, R.F.; Das, R.; Cuppels, M.; Rotello, V.M. Functionalized Polymers Enhance Permeability of Antibiotics in Gram-Negative MDR Bacteria and Biofilms for Synergistic Antimicrobial Therapy. Adv. Ther. 2020, 3, 2000005. [Google Scholar] [CrossRef]
- Nabawy, A.; Makabenta, J.M.; Schmidt-Malan, S.; Park, J.; Li, C.-H.; Huang, R.; Fedeli, S.; Chattopadhyay, A.N.; Patel, R.; Rotello, V.M. Dual Antimicrobial-Loaded Biodegradable Nanoemulsions for Synergistic Treatment of Wound Biofilms. J. Control. Release 2022, 347, 379–388. [Google Scholar] [CrossRef]
- Voytik-Harbin, S.L.; Brightman, A.O.; Waisner, B.; Lamar, C.H.; Badylak, S.F. Application and Evaluation of the Alamarblue Assay for Cell Growth and Survival of Fibroblasts. In Vitr. Cell Dev. Biol. Anim. 1998, 34, 239–246. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Design and Synthesis of Novel Antimicrobial Agents. Antibiotics 2023, 12, 628. [Google Scholar] [CrossRef]
- Mantravadi, P.; Kalesh, K.; Dobson, R.; Hudson, A.; Parthasarathy, A. The Quest for Novel Antimicrobial Compounds: Emerging Trends in Research, Development, and Technologies. Antibiotics 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, Y.; Zaleta-Pinet, D.A.; Borris, R.P.; Clark, B.R. Antibacterial and Anti-Biofilm Activity of Pyrones from a Pseudomonas Mosselii Strain. Antibiotics 2022, 11, 1655. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; Abd El-Hack, M.E. Using Essential Oils to Overcome Bacterial Biofilm Formation and Their Antimicrobial Resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef]
- Landis, R.F.; Li, C.-H.; Gupta, A.; Lee, Y.-W.; Yazdani, M.; Ngernyuang, N.; Altinbasak, I.; Mansoor, S.; Khichi, M.A.S.; Sanyal, A.; et al. Biodegradable Nanocomposite Antimicrobials for the Eradication of Multidrug-Resistant Bacterial Biofilms without Accumulated Resistance. J. Am. Chem. Soc. 2018, 140, 6176–6182. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Madden, L.; Low, S.H.; Phillips, A.R.J.; Kline, K.A.; Becker, D.L. The Effects of Staphylococcus Aureus Biofilm Conditioned Media on 3T3 Fibroblasts. FEMS Microbes 2021, 2, xtab010. [Google Scholar] [CrossRef] [PubMed]
- Kirker, K.R.; James, G.A. In Vitro Studies Evaluating the Effects of Biofilms on Wound-Healing Cells: A Review. APMIS 2017, 125, 344–352. [Google Scholar] [CrossRef] [Green Version]




| Compound | MIC (mg/L) | ||
|---|---|---|---|
| S. aureus | MRSA | B. subtilis | |
| PA1 | 500 | >500 | 500 |
| PA2 | 0.5 | 12.5 | 50 |
| PA3 | 25 | 177 | 50 |
| PA1 | PA2 | PA3 | |
|---|---|---|---|
| Drug concentrations in eugenol (mg/mL) | 48 | 100 | 25 |
| Materials | MBC |
|---|---|
| MRSA (IDRL-6169) | |
| PA2 | 12 mg/L |
| BNE | 16% (v/v) |
| PA-BNE | 8% (v/v), 4 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Mahida, N.; Ho, G.; Pena, E.; Makabenta, J.M.V.; Aneke, S.; Jiang, M.; Bouthillette, L.M.; Holz, S.E.; Hassan, M.A.; et al. Integration of Antimicrobials and Delivery Systems: Synergistic Antibiofilm Activity with Biodegradable Nanoemulsions Incorporating Pseudopyronine Analogs. Antibiotics 2023, 12, 1240. https://doi.org/10.3390/antibiotics12081240
Park J, Mahida N, Ho G, Pena E, Makabenta JMV, Aneke S, Jiang M, Bouthillette LM, Holz SE, Hassan MA, et al. Integration of Antimicrobials and Delivery Systems: Synergistic Antibiofilm Activity with Biodegradable Nanoemulsions Incorporating Pseudopyronine Analogs. Antibiotics. 2023; 12(8):1240. https://doi.org/10.3390/antibiotics12081240
Chicago/Turabian StylePark, Jungmi, Neel Mahida, Gabrielle Ho, Elizabeth Pena, Jessa Marie V. Makabenta, Stanley Aneke, Mingdi Jiang, Leah M. Bouthillette, Stephanie E. Holz, Muhammad Aamir Hassan, and et al. 2023. "Integration of Antimicrobials and Delivery Systems: Synergistic Antibiofilm Activity with Biodegradable Nanoemulsions Incorporating Pseudopyronine Analogs" Antibiotics 12, no. 8: 1240. https://doi.org/10.3390/antibiotics12081240
APA StylePark, J., Mahida, N., Ho, G., Pena, E., Makabenta, J. M. V., Aneke, S., Jiang, M., Bouthillette, L. M., Holz, S. E., Hassan, M. A., Wolfe, A. L., & Rotello, V. M. (2023). Integration of Antimicrobials and Delivery Systems: Synergistic Antibiofilm Activity with Biodegradable Nanoemulsions Incorporating Pseudopyronine Analogs. Antibiotics, 12(8), 1240. https://doi.org/10.3390/antibiotics12081240