Real-World Data about Commonly Used Antibiotics in Long-Term Care Homes in Australia from 2016 to 2019
Abstract
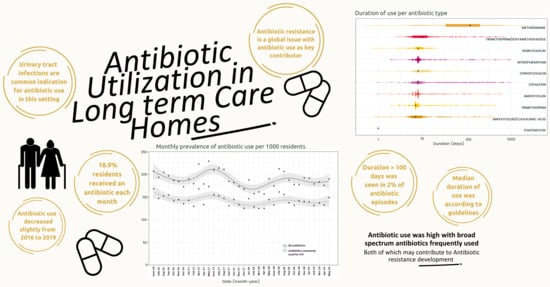
:1. Introduction
2. Results
2.1. Cohort Characteristics
2.2. Monthly Trends of All Systemic Antibiotic Episodes
2.3. Monthly Trends per Individual Antibiotic
2.4. Duration of Use
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Design
4.2. Setting and Population
4.3. Data Source
4.4. Definitions
4.4.1. Antibiotics
4.4.2. Antibiotic Episodes
4.5. Follow-Up Time
4.6. Analysis
4.6.1. Monthly Prevalence of Residents with One or More Antibiotic Episodes
4.6.2. Duration of Antibiotic Use
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassone, M.; Mody, L. Colonization with Multidrug-Resistant Organisms in Nursing Homes: Scope, Importance, and Management. Curr. Geriatr. Rep. 2015, 4, 87–95. [Google Scholar] [CrossRef]
- Hulscher, M.E.J.L.; van der Meer, J.W.M.; Grol, R.P.T.M. Antibiotic use: How to improve it? Int. J. Med. Microbiol. 2010, 300, 351–356. [Google Scholar] [CrossRef]
- Falcone, M.; Paul, M.; Yahav, D.; Orlando, G.; Tiseo, G.; Prendki, V.; Güerri-Fernández, R.; Gavazzi, G.; Mutters, N.T.; Cookson, B.; et al. Antimicrobial consumption and impact of antimicrobial stewardship programmes in long-term care facilities. Clin. Microbiol. Infect. 2019, 25, 562–569. [Google Scholar] [CrossRef]
- Raban, M.Z.; Gates, P.J.; Gasparini, C.; Westbrook, J.I. Temporal and regional trends of antibiotic use in long-term aged care facilities across 39 countries, 1985–2019: Systematic review and meta-analysis. PLoS ONE 2021, 16, e0256501. [Google Scholar] [CrossRef]
- Australian Commission on Safety and Quality in Health Care (ACSQHC). AURA 2019: Third Australian Report on Antimicrobial Use and Resistance in Human Health; ACSQHC: Sydney, Australia, 2019.
- Lim, C.J.; McLellan, S.C.; Cheng, A.C.; Culton, J.M.; Parikh, S.N.; Peleg, A.Y.; Kong, D.C.M. Surveillance of infection burden in residential aged care facilities. Med. J. Aust. 2012, 196, 327–331. [Google Scholar] [CrossRef]
- Alberg, T.; Holen, Ø.; Blix, H.S.; Lindbæk, M.; Bentele, H.; Eriksen, H.M. Antibiotic use and infections in nursing homes. Tidsskr. Nor. Laegeforen. 2017, 137, 357–361. [Google Scholar] [CrossRef]
- Daneman, N.; Gruneir, A.; Newman, A.; Fischer, H.D.; Bronskill, S.E.; Rochon, P.A.; Anderson, G.M.; Bell, C.M. Antibiotic use in long-term care facilities. J. Antimicrob. Chemother. 2011, 66, 2856–2863. [Google Scholar] [CrossRef]
- Smith, M.R.N.; Atkins, S.R.N.B.N.; Worth, L.M.F.P.; Richards, M.M.F.M.D.; Bennett, N.R.N.M.P.H.P. Infections and antimicrobial use in Australian residential aged care facilities: A comparison between local and international prevalence and practices. Aust. Health Rev. 2013, 37, 1–34. [Google Scholar] [CrossRef]
- Lim, C.J.; Kwong, M.; Stuart, R.L.; Buising, K.L.; Friedman, N.D.; Bennett, N.; Cheng, A.C.; Peleg, A.Y.; Marshall, C.; Kong, D.C. Antimicrobial stewardship in residential aged care facilities: Need and readiness assessment. BMC Infect. Dis. 2014, 14, 410. [Google Scholar] [CrossRef]
- van Buul, L.W.; van der Steen, J.T.; Veenhuizen, R.B.; Achterberg, W.P.; Schellevis, F.G.; Essink, R.T.G.M.; van Benthem, B.H.B.; Natsch, S.; Hertogh, C.M.P.M. Antibiotic Use and Resistance in Long Term Care Facilities. J. Am. Med. Dir. Assoc. 2012, 13, 568.e1–568.e13. [Google Scholar] [CrossRef]
- 2023 Therapeutic Guidelines Limited. Urinary Tract Infection in Aged-Care Facility Residents. eTG March 2021 Edition, April 2019. Available online: www.tg.org.au (accessed on 25 May 2022).
- Bergman, J.; Schjøtt, J.; Blix, H.S. Prevention of urinary tract infections in nursing homes: Lack of evidence-based prescription? BMC Geriatr. 2011, 11, 69. [Google Scholar] [CrossRef]
- Thompson, N.D.; Penna, A.; Eure, T.R.; Bamberg, W.M.; Barney, G.; Barter, D.; Clogher, P.; DeSilva, M.B.; Dumyati, G.; Epson, E.; et al. Epidemiology of Antibiotic Use for Urinary Tract Infection in Nursing Home Residents. J. Am. Med. Dir. Assoc. 2020, 21, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Daneman, N.; Gruneir, A.; Bronskill, S.E.; Newman, A.; Fischer, H.D.; Rochon, P.A.; Anderson, G.M.; Bell, C.M. Prolonged Antibiotic Treatment in Long-term Care: Role of the Prescriber. JAMA Intern. Med. 2013, 173, 673–682. [Google Scholar] [CrossRef]
- Sluggett, J.K.; Moldovan, M.; Lynn, D.J.; Papanicolas, L.E.; Crotty, M.; Whitehead, C.; Wesselingh, S.L.; Rogers, G.B.; Inacio, M.C. National Trends in Antibiotic Use in Australian Residential Aged Care Facilities, 2005–2016. Clin. Infect. Dis. 2021, 72, 2167–2174. [Google Scholar] [CrossRef]
- Raban, M.Z.; Lind, K.E.; Day, R.O.; Gray, L.; Georgiou, A.; Westbrook, J.I. Trends, determinants and differences in antibiotic use in 68 residential aged care homes in Australia, 2014–2017: A longitudinal analysis of electronic health record data. BMC Health Serv. Res. 2020, 20, 883. [Google Scholar] [CrossRef]
- Marra, F.; McCabe, M.; Sharma, P.; Zhao, B.; Mill, C.; Leung, V.; Chong, M.; Patrick, D.M. Utilization of Antibiotics in Long-Term Care Facilities in British Columbia, Canada. J. Am. Med. Dir. Assoc. 2017, 18, 1098.e1–1098.e11. [Google Scholar] [CrossRef]
- McClean, P.; Hughes, C.; Tunney, M.; Goossens, H.; Jans, B.; on behalf of the European Surveillance of Antimicrobial Consumption Nursing Home Project Group; Jans, B.; Stroobants, R.; Goossens, H.; Budimir, A.; et al. Antimicrobial prescribing in European nursing homes. J. Antimicrob. Chemother. 2011, 66, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- NPS MedicineWise. Antibiotic Resistance in Australia: Here and Now. Available online: https://www.nps.org.au/news/antibiotic-resistance-in-australia-here-and-now (accessed on 25 May 2022).
- Schjørring, S.; Krogfelt, K.A. Assessment of bacterial antibiotic resistance transfer in the gut. Int. J. Microbiol. 2011, 2011, 312956. [Google Scholar] [CrossRef]
- Department of Health, A.G. Pharmaceutical Benefits Scheme (PBS). Available online: https://www.pbs.gov.au/pbs/home (accessed on 25 May 2022).
- Lim, C.J.; Stuart, R.L.; Kong, D.C.M. Antibiotic use in residential aged care facilities. Aust. Fam. Physician 2015, 44, 192–196. [Google Scholar]
- World Health Organisation. 2021 AWaRe classification. WHO 30 September 2021. Available online: https://www.who.int/publications/i/item/2021-aware-classification (accessed on 28 August 2023).
- Mody, L.; Greene, M.T.; Meddings, J.; Krein, S.L.; McNamara, S.E.; Trautner, B.W.; Ratz, D.; Stone, N.D.; Min, L.; Schweon, S.J.; et al. A National Implementation Project to Prevent Catheter-Associated Urinary Tract Infection in Nursing Home Residents. JAMA Intern. Med. 2017, 177, 1154–1162. [Google Scholar] [CrossRef]
- Kistler, C.E.; Zimmerman, S.; Scales, K.; Ward, K.; Weber, D.; Reed, D.; McClester, M.; Sloane, P.D. The Antibiotic Prescribing Pathway for Presumed Urinary Tract Infections in Nursing Home Residents. J. Am. Geriatr. Soc. 2017, 65, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Admissions into Aged Care; Australian Government: Canberra, Australia, 2019. Available online: https://www.gen-agedcaredata.gov.au/Topics/Admissions-into-aged-care (accessed on 10 August 2023).
- Etty-Leal, M.G. The role of dose administration aids in medication management for older people. J. Pharm. Pract. Res. 2017, 47, 241–247. [Google Scholar] [CrossRef]
- Taxis, K.; Kochen, S.; Wouters, H.; Boersma, F.; Jan Gerard, M.; Mulder, H.; Pavlovic, J.; Stevens, G.; McLachlan, A.; Pont, L.G. Cross-national comparison of medication use in Australian and Dutch nursing homes. Age Ageing 2017, 46, 320–323. [Google Scholar] [CrossRef]
- van der Meer, H.G.; Taxis, K.; Pont, L.G. Changes in Prescribing Symptomatic and Preventive Medications in the Last Year of Life in Older Nursing Home Residents. Front. Pharmacol. 2018, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Gadzhanova, S.; Roughead, E.E.; Pont, L.G. Safety of opioid patch initiation in Australian residential aged care. Med. J. Aust. 2015, 203, 298. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Gadzhanova, S.; Roughead, E.E.; Ward, M.B.; Pont, L.G. The use of antipsychotics among people treated with medications for dementia in residential aged care facilities. Int. Psychogeriatr. 2016, 28, 977–982. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. 2023. Available online: https://www.whocc.no (accessed on 18 July 2023).



| 2016–2017 (n = 1592 Residents) | 2017–2018 (n = 1543 Residents) | 2018–2019 (n = 1487 Residents) | |
|---|---|---|---|
| Number of LTC facilities in cohort | n = 18 | n = 17 | n = 18 |
| Gender | |||
| Females | 63.9 % (n = 1018) | 62.8 % (n = 969) | 62.2% (n = 925) |
| Male | 28.9% (n = 459) | 31.0% (n = 478) | 31.7% (n = 472) |
| Unknown | 7.2% (n = 115) | 6.2% (n = 96) | 6.1% (n = 90) |
| Age, mean (SD) | 85.2 (8.6) | 85.1 (8.9) | 85.2 (8.9) |
| Total follow-up time (Per 1000 residents’ days) | 585.5 | 597.5 | 584.7 |
| Antibiotic Types n = 10,460 | Number of Episodes (%) |
|---|---|
| Cefalexin | 4003 (38.3) |
| Amoxicillin with clavulanic acid | 1190 (11.4) |
| Amoxicillin | 1148 (11.0) |
| Doxycycline | 1024 (9.8) |
| Trimethoprim | 875 (8.4) |
| Flucloxacillin | 570 (5.4) |
| Trimethoprim with sulfamethoxazole | 281 (2.7) |
| Ciprofloxacin | 250 (2.4) |
| Roxithromycin | 250 (2.4) |
| Clindamycin | 232 (2.2) |
| Metronidazole | 109 (1.0) |
| Nitrofurantoin | 104 (1.0) |
| Cefuroxime | 102 (1.0) |
| Clarithromycin | 73 (0.7) |
| Erythromycin | 50 (0.5) |
| Cefaclor | 40 (0.4) |
| Phenoxymethylpenicillin | 38 (0.4) |
| Norfloxacin | 32 (0.3) |
| Methenamine | 29 (0.3) |
| Dicloxacillin | 26 (0.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smit, C.C.H.; Rogers, K.; Robertson, H.; Taxis, K.; Pont, L.G. Real-World Data about Commonly Used Antibiotics in Long-Term Care Homes in Australia from 2016 to 2019. Antibiotics 2023, 12, 1393. https://doi.org/10.3390/antibiotics12091393
Smit CCH, Rogers K, Robertson H, Taxis K, Pont LG. Real-World Data about Commonly Used Antibiotics in Long-Term Care Homes in Australia from 2016 to 2019. Antibiotics. 2023; 12(9):1393. https://doi.org/10.3390/antibiotics12091393
Chicago/Turabian StyleSmit, Chloé C. H., Kris Rogers, Hamish Robertson, Katja Taxis, and Lisa G. Pont. 2023. "Real-World Data about Commonly Used Antibiotics in Long-Term Care Homes in Australia from 2016 to 2019" Antibiotics 12, no. 9: 1393. https://doi.org/10.3390/antibiotics12091393
APA StyleSmit, C. C. H., Rogers, K., Robertson, H., Taxis, K., & Pont, L. G. (2023). Real-World Data about Commonly Used Antibiotics in Long-Term Care Homes in Australia from 2016 to 2019. Antibiotics, 12(9), 1393. https://doi.org/10.3390/antibiotics12091393