Evidence for Anti-Pseudogymnoascus destructans (Pd) Activity of Propolis
Abstract
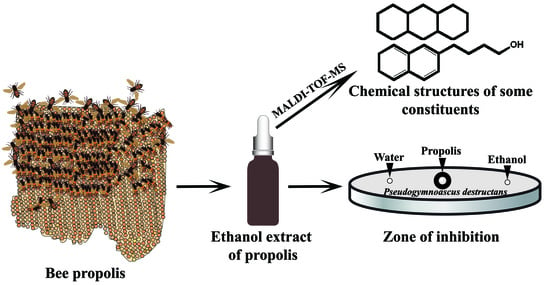
:1. Introduction
2. Results
2.1. Kirby–Bauer Diffusion Assay
2.2. Suppression of Germination of Pd Spores
2.3. Microscopic Examination of the Treated Pd Spores
2.4. Chemical Composition of Propolis
3. Discussion
4. Materials and Methods
4.1. Cultivation of Pd Spores
4.2. Kirby–Bauer Diffusion Assay
4.3. Germule Suppression Assay
4.4. Analysis of Chemical Constituents
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United States Fish and Wildlife Service (USFWS). North American bat death toll exceeds 5.5 million from white-nose syndrome. In USFWS News Release; United States Fish and Wildlife Service (USFWS): Washington, DC, USA, 2012; pp. 1–2. [Google Scholar]
- United States Fish and Wildlife Service (USFWS). White Nose Sydromes: Where Is It Now? In USFWS News Release; United States Fish and Wildlife Service (USFWS): Washington, DC, USA, 2017.
- Verant, M.L.; Boyles, J.G.; Waldrep, W., Jr.; Wibbelt, G.; Blehert, D.S. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS ONE 2012, 7, e46280. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, A.J.; Knudsen, G.M.; Beekman, C.; Perry, J.A.; Johnson, A.D.; DeRisi, J.L.; Craik, C.S.; Bennett, R.J. Destructin-1 is a collagen-degrading endopeptidase secreted by pseudogymnoascus destructans, the causative agent of white-nose syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 7478–7483. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.I.; Speakman, J.R.; Racey, P.A. How hot is a hibernaculum? A review of the temperatures at which bats hibernate. Can. J. Zool. 1996, 74, 761–765. [Google Scholar]
- Davies, W.H. Hibernation: Ecology and physiological ecology. In Biology of Bats; Wimsatt, W.A., Ed.; Acedemic Press: New York, NY, USA; London, UK, 1970; Volume 1, pp. 265–300. [Google Scholar]
- Humphries, M.M.; Thomas, D.W.; Speakman, J.R. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature 2002, 418, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Thomas, D.W. Physiological ecology and enegetics of bats. In Bat Ecology; Kunz, T.H., Fenton, M.B., Eds.; The University of Chicago Press: Chicago, IL, USA; London, UK, 2003; pp. 431–434. [Google Scholar]
- Warnecke, L.; Turner, J.M.; Bollinger, T.K.; Misra, V.; Cryan, P.M.; Blehert, D.S.; Wibbelt, G.; Willis, C.K. Pathophysiology of white-nose syndrome in bats: A mechanistic model linking wing damage to mortality. Biol. Lett. 2013, 9, 20130177. [Google Scholar] [CrossRef] [PubMed]
- Cryan, P.M.; Meteyer, C.U.; Boyles, J.G.; Blehert, D.S. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 2010, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Boyles, J.G.; Cryan, P.M.; McCracken, G.F.; Kunz, T.H. Conservation. Economic importance of bats in agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, C.T.; Keel, M.K.; Gabriel, K.T.; Barlament, C.K.; Tucker, T.A.; Pierce, G.E.; Crow, S.A. A preliminary report on the contact-independent antagonism of Pseudogymnoascus destructans by Rhodococcus rhodochrous strain DAP96253. BMC Microbiol. 2014, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, J.R.; Cheng, T.L.; Langwig, K.E.; Hee, M.M.; Frick, W.F.; Kilpatrick, A.M. Bacteria isolated from bats inhibit the growth of Pseudogymnoascus destructans, the causative agent of white-nose syndrome. PLoS ONE 2015, 10, e0121329. [Google Scholar] [CrossRef] [PubMed]
- Boire, N.; Zhang, S.; Khuvis, J.; Lee, R.; Rivers, J.; Crandall, P.; Keel, M.K.; Parrish, N. Potent inhibition of Pseudogymnoascus destructans, the causative agent of white-nose syndrome in bats, by cold-pressed, terpeneless, valencia orange oil. PLoS ONE 2016, 11, e0148473. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, M.K.; Punekar, S.A.; Ranade, R.V.; Paknikar, K.M. Antimicrobial activity of stingless bee (Trigona sp.) propolis used in the folk medicine of western Maharashtra, India. J. Ethnopharmacol. 2012, 141, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Khacha-ananda, S.; Tragoolpua, K.; Chantawannakul, P.; Tragoolpua, Y. Propolis extracts from the northern region of Thailand suppress cancer cell growth through induction of apoptosis pathways. Investig. New Drugs 2016, 34, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Hepburn, H.R.; Li, Y.; Chen, M.; Radloff, S.E.; Daya, S. Effects of ethanol and water extracts of propolis (bee glue) on acute inflammatory animal models. J. Ethnopharmacol. 2005, 100, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ping, S.; Huang, S.; Hu, L.; Xuan, H.Z.; Zhang, C.P.; Hu, F.L. Molecular mechanisms underlying the in vitro anti-inflammatory effects of a Ffavonoid-rich ethanol extract from Chinese propolis (poplar type). Evid. Based Complement. Altern. Med. 2013, 2013, 127672. [Google Scholar]
- Xuan, H.; Zhao, J.; Miao, J.; Li, Y.; Chu, Y.; Hu, F. Effect of Brazilian propolis on human umbilical vein endothelial cell apoptosis. Food Chem. Toxicol. 2011, 49, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Zhu, R.; Li, Y.; Hu, F. Inhibitory effect of chinese propolis on phosphatidylcholine-specific phospholipase C activity in vascular endothelial cells. Evid. Based Complement. Altern. Med. 2011, 2011, 985278. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Chang, F.R.; Wang, H.K.; Park, Y.K.; Ikegaki, M.; Kilgore, N.; Lee, K.H. Anti-AIDS agents. 48.(1) ANTI-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J. Nat. Prod. 2001, 64, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Teerasripreecha, D.; Phuwapraisirisan, P.; Puthong, S.; Kimura, K.; Okuyama, M.; Mori, H.; Kimura, A.; Chanchao, C. In vitro antiproliferative/cytotoxic activity on cancer cell lines of a cardanol and a cardol enriched from Thai Apis mellifera propolis. BMC Complement. Altern. Med. 2012, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Silici, S.; Kutluca, S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 2005, 99, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Grange, J.M.; Davey, R.W. Antibacterial properties of propolis (bee glue). J. R. Soc. Med. 1990, 83, 159–160. [Google Scholar] [PubMed]
- Shimizu, T.; Takeshita, Y.; Takamori, Y.; Kai, H.; Sawamura, R.; Yoshida, H.; Watanabe, W.; Tsutsumi, A.; Park, Y.K.; Yasukawa, K.; et al. Efficacy of Brazilian propolis against herpes simplex virus type 1 infection in mice and their modes of antiherpetic efficacies. Evid. Based Complement. Altern. Med. 2011, 2011, 976196. [Google Scholar] [CrossRef] [PubMed]
- Silici, S.; Koc, A.N. Comparative study of in vitro methods to analyse the antifungal activity of propolis against yeasts isolated from patients with superficial mycoses. Lett. Appl. Microbiol. 2006, 43, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Amoros, M.; Simoes, C.M.; Girre, L.; Sauvager, F.; Cormier, M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 1992, 55, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Alencar, S.M.; Koo, H.; Ikegaki, M.; Silva, G.V.; Napimoga, M.H.; Rosalen, P.L. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J. Agric. Food Chem. 2013, 61, 4546–4550. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Fernandes-Silva, C.C.; Righi, A.A.; Salatino, M.L. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011, 28, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Buckley, T.R.; Frati, F.; Stewart, J.B.; Beckenbach, A.T. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 545–579. [Google Scholar] [CrossRef]
- Fernandes-Silva, C.C.; Freitas, J.C.; Salatino, A.; Salatino, M.L. Cytotoxic activity of six samples of Brazilian propolis on sea urchin (Lytechinus variegatus) eggs. Evid. Based Complement. Altern. Med. 2013, 2013, 619361. [Google Scholar] [CrossRef] [PubMed]
- McArthur, R.L.; Ghosh, S.; Cheeptham, N. Improvement of protocols for the screening of biological control agents against white-nose syndrome. JEMI+ 2017, 2, 1–7. [Google Scholar]
- Langwig, K.E.; Frick, W.F.; Hoyt, J.R.; Parise, K.L.; Drees, K.P.; Kunz, T.H.; Foster, J.T.; Kilpatrick, A.M. Drivers of variation in species impacts for a multi-host fungal disease of bats. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, C.T.; Gabriel, K.T.; Barlament, C.; Crow, S.A., Jr. Inhibition of Pseudogymnoascus destructans growth from conidia and mycelial extension by bacterially produced volatile organic compounds. Mycopathologia 2014, 177, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Christov, R.; Trusheva, B.; Popova, M.; Bankova, V.; Bertrand, M. Chemical composition of propolis from Canada, its antiradical activity and plant origin. Nat. Prod. Res. 2006, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Recent trends and important developments in propolis research. Evid. Based Complement. Altern. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Todorov, I.; Ninova, M.; Najdenski, H.; Daneshmand, A.; Bankova, V. Antibacterial mono- and sesquiterpene esters of benzoic acids from iranian propolis. Chem. Cent. J. 2010, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Uzel, A.; Sorkun, K.; Oncag, O.; Cogulu, D.; Gencay, O.; Salih, B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Gomes, B.P.; Rosalen, P.L.; Ambrosano, G.M.; Park, Y.K.; Cury, J.A. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch. Oral Biol. 2000, 45, 141–148. [Google Scholar] [CrossRef]
- Santos, F.A.; Bastos, E.M.; Rodrigues, P.H.; de Uzeda, M.; de Carvalho, M.A.; Farias Lde, M.; Moreira, E.S. Susceptibility of Prevotella intermedia/Prevotella nigrescens (and Porphyromonas gingivalis) to propolis (bee glue) and other antimicrobial agents. Anaerobe 2002, 8, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Abu-Seida, A.M. Effect of propolis on experimental cutaneous wound healing in dogs. Vet. Med. Int. 2015, 2015, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Khankhet, J.; Vanderwolf, K.J.; McAlpine, D.F.; McBurney, S.; Overy, D.P.; Slavic, D.; Xu, J. Clonal expansion of the Pseudogymnoascus destructans genotype in North America is accompanied by significant variation in phenotypic expression. PLoS ONE 2014, 9, e104684. [Google Scholar] [CrossRef] [PubMed]




| Peak | Constituents Identified | Mass/Charge (m/z) | Intensity |
|---|---|---|---|
| 1 | Benzyl alcohol | 108.14 | 0.77 |
| 2 | Hydroquinone | 110.11 | 5.00 |
| 3 | Benzoic acid | 122.12 | 5.93 |
| 4 | Cinnamyl alcohol | 134.17 | 23.81 |
| 5 | Hydroxyacetophenone | 136.15 | 7.82 |
| 6 | 4-Hydroxybenzoic acid | 138.12 | 10.98 |
| 7 | Cinnamic acid | 148.16 | 14.81 |
| 8 | p-coumaric acid | 164.16 | 4.91 |
| 9 | 3-Phenyl-3-hydroxypropanoic acid | 166.18 | 5.91 |
| 10 | Sesquiterpenes | 168.31 | 13.90 |
| 11 | Ferulic acid | 194.18 | 3.91 |
| 12 | Benzyl benzoate | 212.25 | 6.85 |
| 13 | Benzyl methoxybenzoate | 242.27 | 211.88 |
| 14 | Benzyl dihydroxybenzoate | 244.24 | 105.16 |
| 15 | Palmitic acid | 256.43 | 64.16 |
| 16 | 2,4,6-Trihydroxydihydrochalcone | 258.27 | 5184.70 |
| 17 | Pinostrobin chalcone | 270.28 | 47.98 |
| 18 | 2,6-Dihydroxy-4-methoxydihydrochalcone | 272.25 | 132.69 |
| 19 | Oleic acid | 282.47 | 28.64 |
| 20 | Stearic acid | 284.31 | 50.69 |
| 21 | Sakuranetin | 286.27 | 44.71 |
| 22 | 2,4,6-Trihydroxy-4-methoxydihydrochalcone | 288.30 | 247.59 |
| 23 | Cinnamyl caffeate | 296.32 | 44.87 |
| 24 | Pinobanksin 3-O-acetate | 314.29 | 2331.83 |
| Parameters | Values |
|---|---|
| Laser | Pulsed nitrogen |
| Laser power | 20–80% |
| Peak selection (mass range) | 40–2000 Da |
| Sample rate | 0.05 GS/s |
| Mass range | Low range |
| Electronic gain | Enhanced 100 mV |
| Realtime smooth | Off |
| Spectrum size | 2069 pts |
| Spectrum delay | 307 pts |
| Laser frequency | 60.0 Hz |
| Laser attenuator offset | 17% |
| Laser attenuator range | 30% |
| Target | MSP 96 target polished steel |
| Matrix | α-cyano-4-hydroxy-cinnamic acid, HCCA |
| Sample | 50% diluted propolis in MeOH covered with 0.1020 M of HCCA in 1:4 (v/v) H2O/acetonitrile |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; McArthur, R.; Guo, Z.C.; McKerchar, R.; Donkor, K.; Xu, J.; Cheeptham, N. Evidence for Anti-Pseudogymnoascus destructans (Pd) Activity of Propolis. Antibiotics 2018, 7, 2. https://doi.org/10.3390/antibiotics7010002
Ghosh S, McArthur R, Guo ZC, McKerchar R, Donkor K, Xu J, Cheeptham N. Evidence for Anti-Pseudogymnoascus destructans (Pd) Activity of Propolis. Antibiotics. 2018; 7(1):2. https://doi.org/10.3390/antibiotics7010002
Chicago/Turabian StyleGhosh, Soumya, Robyn McArthur, Zhi Chao Guo, Rory McKerchar, Kingsley Donkor, Jianping Xu, and Naowarat Cheeptham. 2018. "Evidence for Anti-Pseudogymnoascus destructans (Pd) Activity of Propolis" Antibiotics 7, no. 1: 2. https://doi.org/10.3390/antibiotics7010002
APA StyleGhosh, S., McArthur, R., Guo, Z. C., McKerchar, R., Donkor, K., Xu, J., & Cheeptham, N. (2018). Evidence for Anti-Pseudogymnoascus destructans (Pd) Activity of Propolis. Antibiotics, 7(1), 2. https://doi.org/10.3390/antibiotics7010002