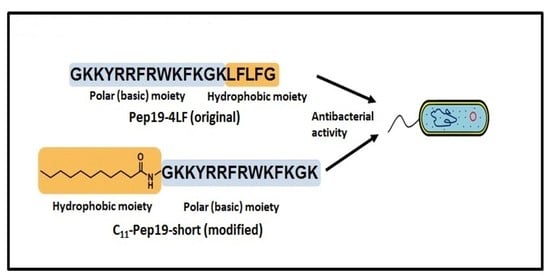
Fatty Acid Conjugation Leads to Length-Dependent Antimicrobial Activity of a Synthetic Antibacterial Peptide (Pep19-4LF)
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Activity
2.2. Hemolysis Assay and Cytotoxicity Assay
2.3. Biodistribution in Wistar Rats
3. Discussion
4. Materials and Methods
4.1. Synthesis of Peptide Conjugates
4.2. Antimicrobial Activity
4.2.1. Minimal Inhibitory Concentration (MIC)
4.2.2. Minimal Bactericidal Concentration (MBC)
4.2.3. Time–Kill Studies
4.3. Hemolysis and Cytotoxicity Assay
4.4. Digestion of C11-Pep19-Short with S9 Fraction from Human Liver
4.5. In Vivo Experiments in Female Wistar Rats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heinbockel, L.; Weindl, G.; Martinez-De-Tejada, G.; Correa, W.; Sanchez-Gomez, S.; Bárcena-Varela, S.; Goldmann, T.; Garidel, P.; Gutsmann, T.; Brandenburg, K. Inhibition of Lipopolysaccharide- and Lipoprotein-Induced Inflammation by Antitoxin Peptide Pep19-2. Front. Immunol. 2018, 9, 1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. The immunopathogenesis of sepsis. Nat. Cell Biol. 2002, 420, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Gutsmann, T.; Razquin-Olazarán, I.; Kowalski, I.; Kaconis, Y.; Howe, J.; Bartels, R.; Hornef, M.; Schuerholz, T.; Roessle, M.; Sanchez-Gómez, S.; et al. New Antiseptic Peptides to Protect against Endotoxin-Mediated Shock. Antimicrob. Agents Chemother. 2010, 54, 3817–3824. [Google Scholar] [CrossRef] [Green Version]
- Heinbockel, L.; Sánchez-Gómez, S.; De Tejada, G.M.; Dömming, S.; Brandenburg, J.; Kaconis, Y.; Hornef, M.; Dupont, A.; Marwitz, S.; Goldmann, T.; et al. Preclinical Investigations Reveal the Broad-Spectrum Neutralizing Activity of Peptide Pep19-2.5 on Bacterial Pathogenicity Factors. Antimicrob. Agents Chemother. 2013, 57, 1480–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfalzgraff, A.; Heinbockel, L.; Su, Q.; Brandenburg, K.; Weindl, G. Synthetic anti-endotoxin peptides inhibit cytoplasmic LPS-mediated responses. Biochem. Pharmacol. 2017, 140, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kaconis, Y.; Kowalski, I.; Howe, J.; Brauser, A.; Richter, W.; Razquin-Olazarán, I.; Iñigo-Pestaña, M.; Garidel, P.; Rössle, M.; De Tejada, G.M.; et al. Biophysical Mechanisms of Endotoxin Neutralization by Cationic Amphiphilic Peptides. Biophys. J. 2011, 100, 2652–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, W.; Heinbockel, L.; Behrends, J.; Kaconis, Y.; Barcena-Varela, S.; Gutsmann, T.; Mauss, K.; Schürholz, T.; Schromm, A.B.; De Tejada, G.M.; et al. Antibacterial action of synthetic antilipopolysaccharide peptides (SALP) involves neutralization of both membrane-bound and free toxins. FEBS J. 2019, 286, 1576–1593. [Google Scholar] [CrossRef]
- Subh, L.; Correa, W.; Pinkvos, T.; Behrens, P.; Brandenburg, K.; Gutsmann, T.; Stiesch, M.; Doll, K.; Winkel, A. Synthetic anti-endotoxin peptides interfere with Gram-positive and Gram-negative bacteria, their adhesion and biofilm formation on titanium. J. Appl. Microbiol. 2020, 129, 1272–1286. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Heinbockel, L.; Su, Q.; Gutsmann, T.; Brandenburg, K.; Weindl, G. Synthetic antimicrobial and LPS-neutralising peptides suppress inflammatory and immune responses in skin cells and promote keratinocyte migration. Sci. Rep. 2016, 6, 31577. [Google Scholar] [CrossRef] [Green Version]
- Kuhlmann, N.; Heinbockel, L.; Correa, W.; Gutsmann, T.; Goldmann, T.; Englisch, U.; Brandenburg, K. Peptide drug stability: The anti-inflammatory drugs Pep19-2.5 and Pep19-4LF in cream formulation. Eur. J. Pharm. Sci. 2018, 115, 240–247. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenbergen, J.N.; Alder, J.; Thorne, G.M.; Tally, F.P. Daptomycin: A lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 2005, 55, 283–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.Y.; Zervos, M.J.; Vazquez, J.A. Dalbavancin: A novel antimicrobial. Int. J. Clin. Pract. 2007, 61, 853–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Feix, J.B. Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 1245–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domhan, C.; Uhl, P.; Meinhardt, A.; Zimmermann, S.; Kleist, C.; Lindner, T.; Leotta, K.; Mier, W.; Wink, M. A novel tool against multiresistant bacterial pathogens: Lipopeptide modification of the natural antimicrobial peptide ranalexin for enhanced antimicrobial activity and improved pharmacokinetics. Int. J. Antimicrob. Agents 2018, 52, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Grimsey, E.; Collis, D.W.; Mikut, R.; Hilpert, K. The effect of lipidation and glycosylation on short cationic antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183195. [Google Scholar] [CrossRef]
- Galbraith, H.; Miller, T.B.; Paton, A.M.; Thompson, J.K. Antibacterial Activity of Long Chain Fatty Acids and the Reversal with Calcium, Magnesium, Ergocalciferol and Cholesterol. J. Appl. Bacteriol. 1971, 34, 803–813. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrimsson, O.; Thormar, H. Killing of Gram-positive cocci by fatty acids and monoglyceridesNote. APMIS 2001, 109, 670–678. [Google Scholar] [CrossRef]
- Mühlberg, E.; Umstätter, F.; Domhan, C.; Hertlein, T.; Ohlsen, K.; Krause, A.; Kleist, C.; Beijer, B.; Zimmermann, S.; Haberkorn, U.; et al. Vancomycin-Lipopeptide Conjugates with High Antimicrobial Activity on Vancomycin-Resistant Enterococci. Pharmaceuticals 2020, 13, 110. [Google Scholar] [CrossRef]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial actions of fatty acids and monoglycerides againstHelicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Jannadi, H.; Correa, W.; Zhang, Z.; Brandenburg, K.; Oueslati, R.; Rouabhia, M. Antimicrobial peptides Pep19–2.5 and Pep19-4LF inhibit Streptococcus mutans growth and biofilm formation. Microb. Pathog. 2019, 133, 103546. [Google Scholar] [CrossRef] [PubMed]
- Schieck, A.; Schulze, A.; Gähler, C.; Müller, T.; Haberkorn, U.; Alexandrov, A.; Urban, S.; Mier, W. Hepatitis B virus hepatotropism is mediated by specific receptor recognition in the liver and not restricted to susceptible hosts. Hepatology 2013, 58, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard—Ninth Edition. CLSI document M07-A9. CLSI 2012, 9, 18–20. [Google Scholar]
- Yeldandi, V.; Strodtman, R.; Lentino, J.R. In-vitro and in-vivo studies of trimethoprim-sulphamethoxazole against multiple resistant Staphylococcus aureus. J. Antimicrob. Chemother. 1988, 22, 873–880. [Google Scholar] [CrossRef]
- Barry, A.L. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999; Volume 19. [Google Scholar]
- Umstätter, F.; Domhan, C.; Hertlein, T.; Ohlsen, K.; Mühlberg, E.; Kleist, C.; Zimmermann, S.; Beijer, B.; Klika, K.D.; Haberkorn, U.; et al. Vancomycin Resistance Is Overcome by Conjugation of Polycationic Peptides. Angew. Chem. Int. Ed. 2020, 59, 8823–8827. [Google Scholar] [CrossRef] [Green Version]
- Crim, J.W.; Garczynski, S.F.; Brown, M.R. Approaches to radioiodination of insect neuropeptides. Peptides 2002, 23, 2045–2051. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storck, P.; Umstätter, F.; Wohlfart, S.; Domhan, C.; Kleist, C.; Werner, J.; Brandenburg, K.; Zimmermann, S.; Haberkorn, U.; Mier, W.; et al. Fatty Acid Conjugation Leads to Length-Dependent Antimicrobial Activity of a Synthetic Antibacterial Peptide (Pep19-4LF). Antibiotics 2020, 9, 844. https://doi.org/10.3390/antibiotics9120844
Storck P, Umstätter F, Wohlfart S, Domhan C, Kleist C, Werner J, Brandenburg K, Zimmermann S, Haberkorn U, Mier W, et al. Fatty Acid Conjugation Leads to Length-Dependent Antimicrobial Activity of a Synthetic Antibacterial Peptide (Pep19-4LF). Antibiotics. 2020; 9(12):844. https://doi.org/10.3390/antibiotics9120844
Chicago/Turabian StyleStorck, Philip, Florian Umstätter, Sabrina Wohlfart, Cornelius Domhan, Christian Kleist, Julia Werner, Klaus Brandenburg, Stefan Zimmermann, Uwe Haberkorn, Walter Mier, and et al. 2020. "Fatty Acid Conjugation Leads to Length-Dependent Antimicrobial Activity of a Synthetic Antibacterial Peptide (Pep19-4LF)" Antibiotics 9, no. 12: 844. https://doi.org/10.3390/antibiotics9120844
APA StyleStorck, P., Umstätter, F., Wohlfart, S., Domhan, C., Kleist, C., Werner, J., Brandenburg, K., Zimmermann, S., Haberkorn, U., Mier, W., & Uhl, P. (2020). Fatty Acid Conjugation Leads to Length-Dependent Antimicrobial Activity of a Synthetic Antibacterial Peptide (Pep19-4LF). Antibiotics, 9(12), 844. https://doi.org/10.3390/antibiotics9120844