Simultaneous Control of Staphylococcus aureus and Bacillus cereus Using a Hybrid Endolysin LysB4EAD-LysSA11
Abstract
:1. Introduction
2. Results and Discussion
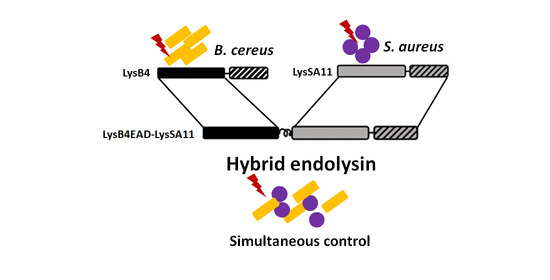
2.1. Construction and Expression of the Hybrid Proteins
2.2. Lytic Activity of the Hybrid Endolysins
2.3. The Antibacterial Spectrum of the Hybrid Proteins
2.4. Thermal Stability Determination
2.5. Effect of pH and NaCl on the Lytic Activity of LysB4EAD-LysSA11
2.6. Antimicrobial Activity of LysB4EAD-LysSA11 in Boiled Rice
3. Materials and Methods
3.1. Bacterial Strains and Growth Conditions
3.2. Construction of Recombinant Proteins
3.3. Protein Expression and Purification
3.4. Lytic Activity Assay
3.5. Effect of pH and Temperature on the Endolysin Activity
3.6. Antimicrobial Activity in Food Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.D.K.; Murali, H.S.; Batra, H.V. Simultaneous detection of pathogenic B. cereus, S. aureus and L. monocytogenes by multiplex PCR. Indian J. Microbiol. 2009, 49, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, R.H.; Sands, E.M.; Friedman, H. Bacillus cereus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Bennett, S.D.; Walsh, K.A.; Gould, L.H. Foodborne Disease Outbreaks Caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus—United States, 1998–2008. Clin. Infect. Dis. 2013, 57, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loessner, M.J. Bacteriophage endolysins—Current state of research and applications. Curr. Opin. Microbiol. 2005, 8, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Futur. Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [Green Version]
- Fischetti, V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Callewaert, L.; Walmagh, M.; Michiels, C.W.; Lavigne, R. Food applications of bacterial cell wall hydrolases. Curr. Opin. Biotechnol. 2011, 22, 164–171. [Google Scholar] [CrossRef]
- Kashani, H.H.; Schmelcher, M.; Sabzalipoor, H.; Hosseini, E.S.; Moniri, R. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: Current status of research and novel delivery strategies. Clin. Microbiol. Rev. 2018, 31, e00071-17. [Google Scholar]
- Gutiérrez, D.; Fernández, L.; Rodríguez, A.; García, P. Are phage lytic proteins the secret weapon to kill Staphylococcus aureus? mBio 2018, 9, e01923-17. [Google Scholar] [CrossRef] [Green Version]
- São-José, C. Engineering of phage-derived lytic enzymes: Improving their potential as antimicrobials. Antibiotics 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, D.M.; Dong, S.; Garrett, W.; Rousseau, G.M.; Moineau, S.; Pritchard, D.G. Peptidoglycan hydrolase fusions maintain their parental specificities. Appl. Environ. Microbiol. 2006, 72, 2988–2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, S.C.; Foster-Frey, J.; Stodola, A.J.; Anacker, D.; Donovan, D.M. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene 2009, 443, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kim, M.; Ryu, S. Characterization of a novel endolysin LysSA11 and its utility as a potent biocontrol agent against Staphylococcus aureus on food and utensils. Food Microbiol. 2017, 68, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Yun, J.; Lim, J.-A.; Shin, H.; Heu, S.; Ryu, S. Characterization of LysB4, an endolysin from the Bacillus cereus-infecting bacteriophage B4. BMC Microbiol. 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, L.Y.; Yang, C.; Perego, M.; Osterman, A.; Liddington, R.C. Structure and lytic activity of a bacillus anthracis prophage endolysin. J. Biol. Chem. 2005, 280, 35433–35439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, L.Y.; Yang, C.; Perego, M.; Osterman, A.; Liddington, R. The role of net charge on the catalytic domain and the influence of the cell-wall binding domain on the bactericidal activity, specificity and host-range of phage lysins. J. Biol. Chem. 2011, 286, 244160. [Google Scholar] [CrossRef] [Green Version]
- Kong, M.; Ryu, S. Bacteriophage PBC1 and its endolysin as an antimicrobial agent against Bacillus cereus. Appl. Environ. Microbiol. 2015, 81, 2274–2283. [Google Scholar] [CrossRef] [Green Version]
- Lavigne, R.; Briers, Y.; Hertveldt, K.; Robben, J.; Volckaert, G. Identification and characterization of a highly thermostable bacteriophage lysozyme. Cell. Mol. Life Sci. 2004, 61, 2753–2759. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Feng, M.G. Bifunctional enhancement of a β-glucanase-xylanase fusion enzyme by optimization of peptide linkers. Appl. Microbiol. Biotechnol. 2008, 79, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Shi, S.; Jiang, Y.; Brink, J.V.D.; De Vries, R.P.; Chen, L.; Zhang, J.; Ma, L.; Wang, C.; Zhou, Z. Construction of a cellulase hyper-expression system in Trichoderma reesei by promoter and enzyme engineering. Microb. Cell Factories 2012, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walkenhorst, W.F.; Klein, J.W.; Vo, P.; Wimley, W.C. The pH dependence of microbe sterilization by cationic antimicrobial peptides: Not just the usual suspects. Antimicrob. Agents Chemother. 2013, 57, 3312–3320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoff, C.; Choudhury, B.; Saile, E.; Quinn, C.P.; Carlson, R.W.; Kannenberg, E.L. Structural elucidation of the nonclassical secondary cell wall polysaccharide from Bacillus cereus ATCC 10987 comparison with the polysaccharides from bacillus anthracis and b. cereus type strain ATCC 14579 reveals both unique and common structural features. J. Biol. Chem. 2008, 283, 29812–29821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillsbury, A.; Chiew, M.; Bates, J.; Sheppeard, V. An outbreak of staphylococcal food poisoning in a commercially catered buffet. Commun. Dis. Intell. 2013, 37, E144–E148. [Google Scholar]
- Grande, M.J.; Lucas, R.; Abriouel, H.; Valdivia, E.; Ben Omar, N.; Maqueda, M.; Martínez-Bueno, M.; Martínez-Cañamero, M.; Gálvez, A. Inhibition of toxicogenic Bacillus cereus in rice-based foods by enterocin AS-48. Int. J. Food Microbiol. 2006, 106, 185–194. [Google Scholar] [CrossRef]
- Fletcher, S.M.; Boonwaat, L.; Moore, T.; Chavada, R.; Conaty, S. Investigating an outbreak of staphylococcal food poisoning among travellers across two Australian states. West. Pac. Surveill. Response J. 2015, 6, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Schmelcher, M.; Loessner, M.J. Bacteriophage endolysins: Applications for food safety. Curr. Opin. Biotechnol. 2016, 37, 76–87. [Google Scholar] [CrossRef]
- Arai, R.; Ueda, H.; Kitayama, A.; Kamiya, N.; Nagamune, T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001, 14, 529–532. [Google Scholar] [CrossRef]
- Nelson, M.D.; Fitch, D.H. Overlap extension PCR: An efficient method for transgene construction. In Molecular Methods for Evolutionary Genetics; Springer: Totowa, NJ, USA, 2012; pp. 459–470. [Google Scholar]





| Lytic Activity a | |||||||
|---|---|---|---|---|---|---|---|
| Strains | LysSA11 | LysSA11-LysB4 | LysB4-LysSA11 | LysSA11-LysB4EAD | LysB4EAD-LysSA11 | LysB4 | |
| S. aureus | ATCC 33586 | + | + | + | + | + | − |
| ATCC 6538 | + | + | + | + | + | − | |
| Newman | + | + | + | + | + | − | |
| RN4220 | + | + | + | + | + | − | |
| ATCC 23235 | + | + | + | + | + | − | |
| ATCC 29213 | + | + | + | + | + | − | |
| ATCC 12600 | + | + | + | + | + | − | |
| ATCC 33593 | + | + | + | + | + | − | |
| ATCC 35983 | + | + | + | + | + | − | |
| ATCC 13301 | + | + | + | + | + | − | |
| CCARM 3793 | + | + | + | + | + | − | |
| CCARM 3090 | + | + | + | + | + | − | |
| S. hominis | ATCC 37844 | + | + | + | + | + | − |
| S. saprophyticus | ATCC 15305 | + | + | + | + | + | − |
| S. heamolyticus | ATCC 29970 | + | + | + | + | + | − |
| S. capitis | ATCC 35661 | + | + | + | + | + | − |
| S. warneri | ATCC 10209 | + | + | + | + | + | − |
| S. xylosis | ATCC29971 | + | + | + | + | + | − |
| S. epidermidis | CCARM 3787 | + | + | + | + | + | − |
| B. cereus | ATCC 21768 | − | + | + | + | + | + |
| B. cereus | ATCC 27348 | − | + | + | + | + | + |
| B. subtilis | 168 | − | + | + | + | + | + |
| L. monocytogenes | ATCC 19114 | − | + | + | + | + | + |
| Plasmids | ||
|---|---|---|
| Descriptions | References | |
| pET28a | Kanr, T7 promoter, His-tagged expression vector | Novagen, Wisconsin, SA |
| pET15b-LysB4 | pE15b with LSA12CBD | [16] |
| pET29b-LysSA11 | pET29a with LSA97CBD | [15] |
| Primers (5′→3′) | ||
| Sequences | ||
| B4EAD_HL_overl_R | TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TCC ACC TGT AGA GCC ACC TCC | |
| SA11EAD_Sal1_R | TTT GTC GAC TTG TAC CTC GTC TTT GAA ATT AGG | |
| SA11EAD_HL_overl_R | TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTG TAC CTC GTC TTT GAA ATT AGG | |
| B4EAD_Sal1_R | TTT GTC GAC TCC ACC TGT AGA GCC ACC TCC | |
| BamH1_B4_F | AAA GGA TCC ATG GCA ATG GCA TTA CAA ACT T | |
| B4_HL_overl_R | TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTT GAA CGT ACC CCA GTA GTT C | |
| SA11_Sal1_R | TTT GTC GAC TTT CCA GTT AAT ACG ACC CCA A | |
| BamH1_SA11_F | AAA GGA TCC ATG AAA GCA TCG ATG ACT AGA A | |
| SA11_HL_overl_R | TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTT TGC CGC AGC TTC TTT CCA GTT AAT ACG ACC CCA A | |
| HL_B4_overl_F | GAA GCT GCG GCA AAA GAA GCT GCG GCA AAA GAA GCT GCG GCA AAA GAA GCT GCG GCA AAA ATG GCA ATG GCA TTA CAA ACT TT | |
| B4_Sal1_R | TTT GTC GAC TTT GAA CGT ACC CCA GTA GTT C | |
| Figure # | Target Bacteria | Observation |
|---|---|---|
| Figure 3A | S. aureus | LysB4EAD-LysSA11 is stable up to 45 °C while LysSA11 is stable up to 37 °C. |
| Figure 3B | B. cereus | LysB4EAD-LysSA11 is stable up to 65 °Cwhile LysB4 is stable up to 55 °C. |
| Figure 4A | S. aureus | LysB4EAD-LysSA11 is highly active at pH 8.0–9.0. |
| Figure 4B | B. cereus | LysB4EAD-LysSA11 is highly active at pH 5.0–9.0. |
| Figure 4C | S. aureus | The lytic activity of LysB4EAD-LysSA11 decreases in the absence of 50 mM NaCl. |
| Figure 4D | B. cereus | The lytic activity of LysB4EAD-LysSA11 decreases in the absence of 50 mM NaCl. |
| Figure 5A,C | S. aureus | 3.0 μM of LysB4EAD-LysSA11 eliminates all bacterial cells in the boiled rice within 2 h while LysSA11 and LyB4EAD in combination (3.0 μM each) eliminates all bacterial cells within 1 h. |
| Figure 5B,D | B. cereus | 3.0 μM of LysB4EAD-LysSA11 eliminates all bacterial cells in the boiled rice within 1 h while LysSA11 and LyB4EAD in combination (3.0 μM each) eliminates 1 log CFU/mL of bacterial cells within 3 h. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, B.; Kong, M.; Cha, Y.; Bai, J.; Ryu, S. Simultaneous Control of Staphylococcus aureus and Bacillus cereus Using a Hybrid Endolysin LysB4EAD-LysSA11. Antibiotics 2020, 9, 906. https://doi.org/10.3390/antibiotics9120906
Son B, Kong M, Cha Y, Bai J, Ryu S. Simultaneous Control of Staphylococcus aureus and Bacillus cereus Using a Hybrid Endolysin LysB4EAD-LysSA11. Antibiotics. 2020; 9(12):906. https://doi.org/10.3390/antibiotics9120906
Chicago/Turabian StyleSon, Bokyung, Minsuk Kong, Yoyeon Cha, Jaewoo Bai, and Sangryeol Ryu. 2020. "Simultaneous Control of Staphylococcus aureus and Bacillus cereus Using a Hybrid Endolysin LysB4EAD-LysSA11" Antibiotics 9, no. 12: 906. https://doi.org/10.3390/antibiotics9120906
APA StyleSon, B., Kong, M., Cha, Y., Bai, J., & Ryu, S. (2020). Simultaneous Control of Staphylococcus aureus and Bacillus cereus Using a Hybrid Endolysin LysB4EAD-LysSA11. Antibiotics, 9(12), 906. https://doi.org/10.3390/antibiotics9120906