Role of Tobramycin in the Induction and Maintenance of Viable but Non-Culturable Pseudomonas aeruginosa in an In Vitro Biofilm Model
Abstract
:1. Introduction
2. Results
2.1. Assessment of Antibiotic Susceptibility
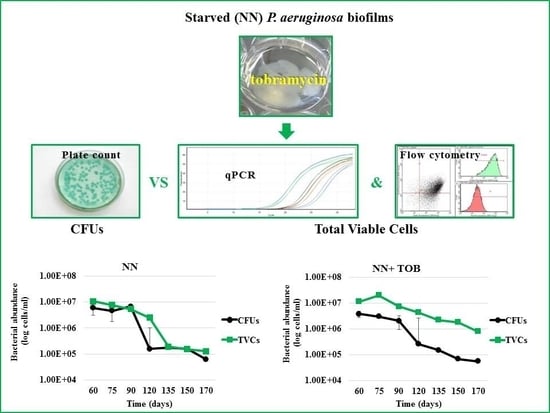
2.2. Stress Exposure of P. aeruginosa Biofilms and VBNC Cells Detection
2.3. Strain-and Antibiotic-Dependent Abundance of VBNC P. aeruginosa Cells
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Growth Media and Chemicals
4.2. Antibiotic Susceptibility Test
4.3. In Vitro P. aeruginosa Biofilm Development and Monitoring
4.4. Biofilm Quantification
4.5. P. aeruginosa CFUs, qPCR and Flow Cytometry Counts
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoo, Z.H.; Edenborough, F.P.; Curley, R.; Prtak, L.; Dewar, J.; Allenby, M.I.; Nightingale, J.A.; Wildman, M.J. Understanding Pseudomonas status among adults with cystic fibrosis: A real-world comparison of the Leeds criteria against clinicians’ decision. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Yoon, S.S. Pseudomonas aeruginosa Biofilm, a Programmed Bacterial Life for Fitness. J. Microbiol. Biotechnol. 2017, 27, 1053–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnane, B.; Kearse, L.; O’Connell, N.H.; Fenton, J.; Kiernan, M.G.; Dunne, C.P. A case of failed eradication of cystic fibrosis-related sinus colonisation by Pseudomonas aeruginosa. BMC Pulm Med. 2015, 15, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, K. Persister cells: Molecular mechanisms related to antibiotic tolerance. Handb. Exp. Pharm. 2012, 211, 121–133. [Google Scholar]
- Ayrapetyan, M.; Williams, T.C.; Oliver, J.D. Bridging the gap between viable but non-culturable and antibiotic persistent bacteria. Trends Microbiol. 2015, 23, 7–13. [Google Scholar] [CrossRef]
- Deschaght, P.; Schelstraete, P.; Van Simaey, L.; Vanderkercken, M.; Raman, A.; Mahieu, L.; Van Daele, S.; De Baets, F.; Vaneechoutte, M. Is the improvement of CF patients, hospitalized for pulmonary exacerbation, correlated to a decrease in bacterial load? PLoS ONE 2013, 8, e79010. [Google Scholar] [CrossRef] [Green Version]
- Le Gall, F.; Le Berre, R.; Rosec, S.; Hardy, J.; Gouriou, S.; Boisramé-Gastrin, S.; Vallet, S.; Rault, G.; Payan, C.; Héry-Arnaud, G. Proposal of a quantitative PCR-based protocol for an optimal Pseudomonas aeruginosa detection in patients with cystic fibrosis. BMC Microbiol. 2013, 13, 143. [Google Scholar] [CrossRef] [Green Version]
- Mangiaterra, G.; Amiri, M.; Di Cesare, A.; Pasquaroli, S.; Manso, E.; Cirilli, N.; Citterio, B.; Vignaroli, C.; Biavasco, F. Detection of viable but non-culturable Pseudomonas aeruginosa in cystic fibrosis by qPCR: A validation study. BMC Infect. Dis. 2018, 18, 701. [Google Scholar] [CrossRef]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehlet, F. Clinical Impact of Antibiotics for the Treatment of Pseudomonas aeruginosa Biofilm Infections. Front. Microbiol. 2020, 10, 2894. [Google Scholar] [CrossRef]
- Davies, J.; Spiegelman, G.B.; Yim, G. The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 2006, 9, 445–453. [Google Scholar] [CrossRef]
- Pasquaroli, S.; Zandri, G.; Vignaroli, C.; Vuotto, C.; Donelli, G.; Biavasco, F. Antibiotic pressure can induce the viable but non-culturable state in Staphylococcus aureus growing in biofilms. J. Antimicrob. Chemother. 2013, 68, 1812–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Bae, S. Molecular viability testing of viable but non-culturable bacteria induced by antibiotic exposure. Microb. Biotechnol. 2018, 11, 1008–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanojevic, S.; Waters, V.; Mathew, J.L.; Taylor, L.; Ratjen, F. Effectiveness of inhaled tobramycin in eradicating Pseudomonas aeruginosa in children with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Al-Obaidi, H.; Kalgudi, R.; Zariwala, M.G. Fabrication of inhaled hybrid silver/ciprofloxacin nanoparticles with synergetic effect against Pseudomonas Aeruginosa. Eur J. Pharm Biopharm. 2018, 128, 27–35. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas Aeruginosa. Protein Cell. 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Mulcahy, L.R.; Burns, J.L.; Lory, S.; Lewis, K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 2010, 192, 6191–6199. [Google Scholar] [CrossRef] [Green Version]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definition of guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial persistence as a phenotypic switch. Sci. Cit. Index 2004, 305, 1622–1625. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayrapetyan, M.; Williams, T.C.; Baxter, R.; Oliver, J.D. Viable but nonculturable and persister cells coexist stochastically and are induced by human serum. Infect. Immun. 2015, 83, 4194–4203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016, 7, 11220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalhais, V.; Pérez-Cabezas, B.; Oliveira, C.; Vitorino, R.; Vilanova, M.; Cerca, N. Tetracycline and rifampicin induced a viable nonculturable state in Staphylococcus epidermidis biofilms. Future Microbiol. 2018, 13, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-seventh Informational Supplement M100-S27; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M.R.; Dinarvand, R. Optimization of tetrazolium salt assay for Pseudomonas aeruginosa biofilm using microtiter plate method. J. Microbiol. Methods. 2014, 105, 134–140. [Google Scholar] [CrossRef]
- Pasquaroli, S.; Citterio, B.; Mangiaterra, G.; Biavasco, F.; Vignaroli, C. Influence of Sublethal Concentrations of Vancomycin and Quinupristin/Dalfopristin on the Persistence of Viable but Non-Culturable Staphylococcus aureus Growing in Biofilms. J. Antimicrob. Chemother. 2018, 73, 3526–3529. [Google Scholar] [CrossRef]
- Camilli, R.; Pantosti, A.; Baldassarri, L. Contribution of serotype and genetic background to biofilm formation by Streptococcus pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 97–102. [Google Scholar] [CrossRef]
- Reyneke, B.; Ndlovu, T.; Khan, S.; Khan, W. Comparison of EMA-, PMA- and DNase qPCR for the determination of microbial cell viability. Appl Microbiol. Biotechnol. 2017, 101, 7371–7383. [Google Scholar] [CrossRef]
- Zandri, G.; Pasquaroli, S.; Vignaroli, C.; Talevi, S.; Manso, E.; Donelli, G.; Biavasco, F. Detection of viable but non-culturable staphylococci in biofilms from central venous catheters negative on standard microbiological assays. Clin. Microbiol. Infect. 2012, 18, E259–E261. [Google Scholar] [CrossRef] [Green Version]
- Wood, T.K.; Song, S.; Yamasaki, R. Ribosome dependence of persister cell formation and resuscitation. J. Microbiol. 2019, 57, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Héry-Arnaud, G.; Nowak, E.; Caillon, J.; David, V.; Dirou, A.; Revert, K.; Munck, M.R.; Frachon, I.; Haloun, A.; Horeau-Langlard, D.; et al. Evaluation of quantitative PCR for early diagnosis of Pseudomonas aeruginosa infection in cystic fibrosis: A prospective cohort study. Clin. Microbiol. Infect. 2017, 23, 203–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the bacterial strains are available from the authors. |


| Strain | Time (Days) | TVCs (VBNC%) | ||
|---|---|---|---|---|
| NN | NN + CIP | NN + TOB | ||
| PAO1-N | 60 | 9.51 × 107 ± 0.13 (97) | 2.85 × 107 ± 0.23 (85.1) | 2.98 × 107 ± 0.14 (93.4) |
| 75 | 3.41 × 107 ± 0.84 (91.6) | 2.76 × 107 ± 0.48 (89.1) | 3.18 × 107 ± 0.50 (98.5 *) | |
| 90 | 5.39 × 107 ± 1.06 (91) | 3.46 × 107 ± 0.80 (94.2) | 3.29 × 107 ± 0.04(98.5) | |
| 120 | 2.45 × 108 ± 0.56 (98.6) | 2.37 × 108 ± 0.06 (99.5) | 2.32 × 108 ± 0.75 (99.7 **) | |
| 135 | 9.43 × 107 ± 0.00 (89) | 1.53 × 108 ± 0.00 (88) | 9.20 × 107 ± 0.00 (99.3 *) | |
| 150 | 1.46 × 108 ± 0.00 (70) | 9.86 × 107 ± 0.00 (88.1 **) | 8.98 × 107 ± 1.73 (98.8 **) | |
| 170 | 2.26 × 108 ± 0.00 (67.7) | 2.05 × 108 ± 0.00 (73.2) | 1.83 × 108 ± 0.63 (97.2 **) | |
| C24 | 60 | 1.07 × 107 ± 0.09 (44.8) | 2.21 × 106 ± 0.73 (ND) | 1.12 × 107 ± 0.01 (66.1) |
| 75 | 7.68 × 106 ± 0.53 (69.7) | 5.31 × 106 ± 1.06 (75.5) | 1.96 × 107 ± 0.02 (84.7 **) | |
| 90 | 5.35 × 106 ± 0.00 (ND) | 3.35 × 106 ± 0.86 (40.3) | 7.35 × 106 ± 0.07(72.1 **) | |
| 120 | 2.49 × 106 ± 0.30 (93.8) | 7.85 × 106 ± 0.45 (96.4 **) | 4.29 × 106 ± 2.10 (94 **) | |
| 135 | 1.93 × 105 ± 0.31 (ND) | 1.05 × 106 ± 0.00 (72.4 **) | 2.21 × 106 ± 0.55 (93.2 **) | |
| 150 | 1.51 × 105 ± 0.44 (ND) | 3.58 × 105 ± 0.79 (23.2 **) | 1.80 × 106 ± 0.06 (62.1 **) | |
| 170 | 1.27 × 105 ± 0.06 (51.1) | 4.57 × 105 ± 0.33 (38.7) | 7.84 × 105 ± 0.61 (93 **) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangiaterra, G.; Cedraro, N.; Vaiasicca, S.; Citterio, B.; Galeazzi, R.; Laudadio, E.; Mobbili, G.; Minnelli, C.; Bizzaro, D.; Biavasco, F. Role of Tobramycin in the Induction and Maintenance of Viable but Non-Culturable Pseudomonas aeruginosa in an In Vitro Biofilm Model. Antibiotics 2020, 9, 399. https://doi.org/10.3390/antibiotics9070399
Mangiaterra G, Cedraro N, Vaiasicca S, Citterio B, Galeazzi R, Laudadio E, Mobbili G, Minnelli C, Bizzaro D, Biavasco F. Role of Tobramycin in the Induction and Maintenance of Viable but Non-Culturable Pseudomonas aeruginosa in an In Vitro Biofilm Model. Antibiotics. 2020; 9(7):399. https://doi.org/10.3390/antibiotics9070399
Chicago/Turabian StyleMangiaterra, Gianmarco, Nicholas Cedraro, Salvatore Vaiasicca, Barbara Citterio, Roberta Galeazzi, Emiliano Laudadio, Giovanna Mobbili, Cristina Minnelli, Davide Bizzaro, and Francesca Biavasco. 2020. "Role of Tobramycin in the Induction and Maintenance of Viable but Non-Culturable Pseudomonas aeruginosa in an In Vitro Biofilm Model" Antibiotics 9, no. 7: 399. https://doi.org/10.3390/antibiotics9070399
APA StyleMangiaterra, G., Cedraro, N., Vaiasicca, S., Citterio, B., Galeazzi, R., Laudadio, E., Mobbili, G., Minnelli, C., Bizzaro, D., & Biavasco, F. (2020). Role of Tobramycin in the Induction and Maintenance of Viable but Non-Culturable Pseudomonas aeruginosa in an In Vitro Biofilm Model. Antibiotics, 9(7), 399. https://doi.org/10.3390/antibiotics9070399