Insulating Thermal and Water-Resistant Hybrid Coating for Fabrics
Abstract
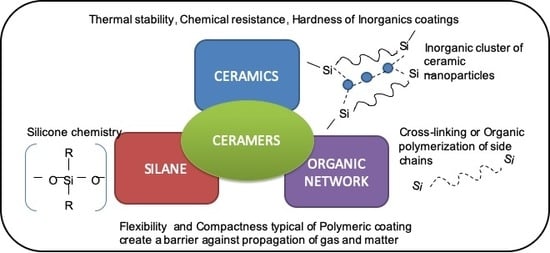
:1. Introduction
2. Experimental
2.1. Materials
2.2. Ceramer Coating Formation
2.3. Characterization of Ceramer Suspensions
2.4. Characterization of Ceramer Powders
2.5. Characterization of Ceramer Coated Fabrics
3. Results and Discussion
3.1. Ceramer Suspensions
3.2. Ceramer Powders
3.3. Ceramer Coating
4. Conclusions
- An environmentally friendly and easily scalable, room temperature, sol–gel process was optimized to apply a ceramer coating on polyester nonwoven fabrics;
- Different ceramer coatings showing an improved thermal stability and enhanced water repellency properties were obtained;
- The results of burn-out tests of ceramised fabrics pointed out that TMEOS precursor characterized by short alkoxy chain length and three hydrolysable groups promotes the formation of the more compact silica-based network, with a homogeneous distribution of elements involved;
- An optimal amount of TiO2 NPs improved the fabrics thermal physical barrier without catalyzing and accelerating the combustion process;
- The addition of P-based compounds in the ceramer formulation improves the thermal stability, whilst the presence of amino groups and their degradation during thermal treatment seems to hinder the formation of a stable and protective –Si-O-Si– network;
- The performances achieved in terms of thermal stability (burn-out residue at 800 °C) were correlated with physicochemical properties of ceramer formulations (composition, FT-IR, and TGA profiles), and a common trend was found between TGA weight losses and burn-out residues;
- The best compromise (highest burn-out residue) was obtained using TMEOS precursor, with an intermediate amount of TiO2 NPs and in the presence of P-based compound;
- The information collected will support the design of new “green” flame retardant and water repellency solutions, which are easily applicable to nonwoven polyester fabrics and usually show a poor affinity for water-based finishing treatments.
Author Contributions
Funding
Conflicts of Interest
References
- Judeinstein, P.; Sanchez, C. Hybrid organic–inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Yano, S.; Iwata, K.; Kurita, K. Physical properties and structure of organic–inorganic hybrid materials produced by sol–gel process. Mater. Sci. Eng. C 1998, 6, 75–90. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/Inorganic Hybrid Network Materials by the Sol−Gel Approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Nazari, M.H.; Shi, X. Polymer-Based Nanocomposite Coatings for Anticorrosion Applications. In Industrial Applications for Intelligent Polymers and Coatings; Hosseini, M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 373–398. ISBN 978-3-319-26891-0. [Google Scholar]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Zare, Y.; Kiany, P. Nanoparticles as Effective Flame Retardants for Natural and Synthetic Textile Polymers: Application, Mechanism, and Optimization. Polym. Rev. 2015, 55, 531–560. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- Noman, M.T.; Militky, J.; Wiener, J.; Saskova, J.; Ashraf, M.A.; Jamshaid, H.; Azeem, M. Sonochemical synthesis of highly crystalline photocatalyst for industrial applications. Ultrasonics 2018, 83, 203–213. [Google Scholar] [CrossRef]
- Noman, M.T.; Wiener, J.; Saskova, J.; Ashraf, M.A.; Vikova, M.; Jamshaid, H.; Kejzlar, P. In-situ development of highly photocatalytic multifunctional nanocomposites by ultrasonic acoustic method. Ultrason. Sonochem. 2018, 40, 41–56. [Google Scholar] [CrossRef]
- Wang, X.Y.; Gong, R.H.; Dong, Z.; Porat, I. Abrasion resistance of thermally bonded 3D nonwoven fabrics. Wear 2007, 262, 424–431. [Google Scholar] [CrossRef]
- Rwei, S.-P.; Jue, Z.-F.; Chen, F.L. PBT/PET conjugated fibers: Melt spinning, fiber properties, and thermal bonding. Polym. Eng. Sci. 2004, 44, 331–344. [Google Scholar] [CrossRef]
- Ghosh, S.; Villarreal, L. Creating aesthetics and functional values in cotton fabrics through the introduction of thermobonding amorphous polyester fibers into blends. J. Appl. Polym. Sci. 2003, 89, 3747–3756. [Google Scholar] [CrossRef]
- Lin, J.H.; Lou, C.W.; Lei, C.H.; Lin, C.Y. Processing conditions of abrasion and heat resistance for hybrid needle-punched nonwoven bag filters. Compos. Part Appl. Sci. Manuf. 2006, 37, 31–37. [Google Scholar] [CrossRef]
- Schnipper, A.; Smith-hansen, L.; Thomsen, E.S. Reduced combustion efficiency of chlorinated compounds, resulting in higher yields of carbon monoxide. Fire Mater. 1995, 19, 61–64. [Google Scholar] [CrossRef]
- Cireli, A.; Onar, N.; Ebeoglugil, M.F.; Kayatekin, I.; Kutlu, B.; Culha, O.; Celik, E. Development of flame retardancy properties of new halogen-free phosphorous doped SiO2 thin films on fabrics. J. Appl. Polym. Sci. 2007, 105, 3748–3756. [Google Scholar] [CrossRef]
- EC Directive 2002/96/EC on Waste of Electric and Electronic Equipment. Off. J Eur Union 2003, 37, 24–38.
- EC Directive 2002/95/EC on Restriction of certain hazardous Substances in Electric and Electronic Equipment. Off. J. Eur. Union 2003, 37, 19–23.
- Yaman, N. Preparation and flammability properties of hybrid materials containing phosphorous compounds via sol–gel process. Fibers Polym. 2009, 10, 413–418. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent Developments in Halogen Free Flame Retardants for Epoxy Resins for Electrical and Electronic Applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef] [Green Version]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Toniazzo, V.; Ruch, D. Polyallylamine–montmorillonite as super flame retardant coating assemblies by layer by layer deposition on polyamide. Polym. Degrad. Stab. 2013, 98, 627–634. [Google Scholar] [CrossRef]
- Qiu, X.; Li, Z.; Li, X.; Zhang, Z. Flame retardant coatings prepared using layer by layer assembly: A review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar] [CrossRef]
- Hornsby, P.R. The Application of Fire-Retardant Fillers for Use in Textile Barrier Materials. In Multifunctional Barriers for Flexible Structure; Duquesne, S., Magniez, C., Camino, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 97, pp. 3–22. ISBN 978-3-540-71917-5. [Google Scholar]
- Kari-Heinz Haas, K. Rose Hybrid inorganic/organic polymers with nanoscale building blocks: Precursors, processing, properties and applications. Rev. Adv. Mater. Sci. 2003, 5, 47–52. [Google Scholar]
- Qian, X.; Song, L.; Hu, Y.; Yuen, R.K.K. Preparation and thermal properties of novel organic/inorganic network hybrid materials containing silicon and phosphate. J. Polym. Res. 2012, 19, 9890. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, Y.; Sun, J. Intumescent Flame-Retardant and Self-Healing Superhydrophobic Coatings on Cotton Fabric. ACS Nano 2015, 9, 4070–4076. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, A.R. Flame retardant challenges for textiles and fibres: New chemistry versus innovatory solutions. Polym. Degrad. Stab. 2011, 96, 377–392. [Google Scholar] [CrossRef]
- Gao, S.; Shi, Y.; Zhang, S.; Jiang, K.; Yang, S.; Li, Z.; Takayama-Muromachi, E. Biopolymer-Assisted Green Synthesis of Iron Oxide Nanoparticles and Their Magnetic Properties. J. Phys. Chem. C 2008, 112, 10398–10401. [Google Scholar] [CrossRef]
- Lewin, M. Reflections on migration of clay and structural changes in nanocomposites. Polym. Adv. Technol. 2006, 17, 758–763. [Google Scholar] [CrossRef]
- Tang, Y.; Lewin, M. New aspects of migration and flame retardancy in polymer nanocomposites. Polym. Degrad. Stab. 2008, 93, 1986–1995. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chou, C.-I. The effect of silicon sources on the mechanism of phosphorus–silicon synergism of flame retardation of epoxy resins. Polym. Degrad. Stab. 2005, 90, 515–522. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Gilman, J.W.; Butler, K.M.; Harris, R.H.; Shields, J.R.; Asano, A. Flame retardant mechanism of silica gel/silica. Fire Mater. 2000, 24, 277–289. [Google Scholar] [CrossRef]
- Lewin, M. Some comments on the modes of action of nanocomposites in the flame retardancy of polymers. Fire Mater. 2003, 27, 1–7. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Guan, J.-P.; Yang, X.-H.; Tang, R.-C. Improvement of flame retardancy of silk fabric by bio-based phytic acid, nano-TiO2, and polycarboxylic acid. Prog. Org. Coat. 2017, 112, 18–26. [Google Scholar] [CrossRef]
- Mascia, L. Developments in Organic–inorganic Polymeric Hybrids: Ceramers. Trends Polym. Sci. 1995, 3, 61–66. [Google Scholar]
- Mishra, A.K.; Allauddin, S.; Narayan, R.; Aminabhavi, T.M.; Raju, K.V.S.N. Characterization of surface-modified montmorillonite nanocomposites. Ceram. Int. 2012, 38, 929–934. [Google Scholar] [CrossRef]
- Gou, W.; Che, X.; Yu, X.; Zhang, F.; An, T.; Sun, Y.; Chen, K. Facile fabrication of waterborne fabric coatings with multifunctional superhydrophobicity and thermal insulation. Mater. Lett. 2019, 250, 123–126. [Google Scholar] [CrossRef]
- Nicole, L.; Laberty-Robert, C.; Rozes, L.; Sanchez, C. Hybrid materials science: A promised land for the integrative design of multifunctional materials. Nanoscale 2014, 6, 6267–6292. [Google Scholar] [CrossRef]
- Malucelli, G. Hybrid Organic/Inorganic Coatings Through Dual-Cure Processes: State of the Art and Perspectives. Coatings 2016, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Ortelli, S.; Malucelli, G.; Cuttica, F.; Blosi, M.; Zanoni, I.; Costa, A.L. Coatings made of proteins adsorbed on TiO2 nanoparticles: A new flame retardant approach for cotton fabrics. Cellulose 2018, 25, 2755–2765. [Google Scholar] [CrossRef]
- Ortelli, S.; Malucelli, G.; Blosi, M.; Zanoni, I.; Costa, A.L. NanoTiO2@DNA complex: A novel eco, durable, fire retardant design strategy for cotton textiles. J. Colloid Interface Sci. 2019, 546, 174–183. [Google Scholar] [CrossRef]
- Ortelli, S.; Costa, A.L. Nanoencapsulation techniques as a “safer by (molecular) design” tool. Nano-Struct. Nano-Objects 2018, 13, 155–162. [Google Scholar] [CrossRef]
- Zhao, J.; Milanova, M.; Warmoeskerken, M.M.C.G.; Dutschk, V. Surface modification of TiO2 nanoparticles with silane coupling agents. Colloids Surf. Physicochem. Eng. Asp. 2012, 413, 273–279. [Google Scholar] [CrossRef]
- Luan, Z.; Fournier, J.A.; Wooten, J.B.; Miser, D.E. Preparation and characterization of (3-aminopropyl)triethoxysilane-modified mesoporous SBA-15 silica molecular sieves. Microporous Mesoporous Mater. 2005, 83, 150–158. [Google Scholar] [CrossRef]
- Ortelli, S.; Costa, A.; Dondi, M. TiO2 Nanosols Applied Directly on Textiles Using Different Purification Treatments. Materials 2015, 8, 7988–7996. [Google Scholar] [CrossRef] [Green Version]
- Ortelli, S.; Poland, C.A.; Baldi, G.; Costa, A.L. Silica matrix encapsulation as a strategy to control ROS production while preserving photoreactivity in nano-TiO2. Environ. Sci. Nano 2016, 3, 602–610. [Google Scholar] [CrossRef]
- Mandile, A.J.; Hutton, A.C. Quantitative X-ray diffraction analysis of mineral and organic phases in organic-rich rocks. Int. J. Coal Geol. 1995, 28, 51–69. [Google Scholar] [CrossRef]
- Edathazhe, A.; Shashikala, H.D. Effect of BaO addition on the structural and mechanical properties of soda lime phosphate glasses. Mater. Chem. Phys. 2016, 184, 146–154. [Google Scholar] [CrossRef]
- Allauddin, S.; Narayan, R.; Raju, K.V.S.N. Synthesis and Properties of Alkoxysilane Castor Oil and Their Polyurethane/Urea–Silica Hybrid Coating Films. ACS Sustain. Chem. Eng. 2013, 1, 910–918. [Google Scholar] [CrossRef]
- Zhang, D.; Williams, B.L.; Shrestha, S.B.; Nasir, Z.; Becher, E.M.; Lofink, B.J.; Santos, V.H.; Patel, H.; Peng, X.; Sun, L. Flame retardant and hydrophobic coatings on cotton fabrics via sol–gel and self-assembly techniques. J. Colloid Interface Sci. 2017, 505, 892–899. [Google Scholar] [CrossRef]
- Xiu, Y.; Hess, D.W.; Wong, C.P. UV and thermally stable superhydrophobic coatings from sol–gel processing. J. Colloid Interface Sci. 2008, 326, 465–470. [Google Scholar] [CrossRef]
- Mohamed, A.L.; El-Sheikh, M.A.; Waly, A.I. Enhancement of flame retardancy and water repellency properties of cotton fabrics using silanol based nano composites. Carbohydr. Polym. 2014, 102, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Pang, Y.; Jiang, X.; Huang, J.; Xi, F.; Liu, J. One-step fabrication of novel superhydrophobic and superoleophilic sponge with outstanding absorbency and flame-retardancy for the selective removal of oily organic solvent from water. Appl. Surf. Sci. 2018, 428, 338–347. [Google Scholar] [CrossRef]
- Bae, G.Y.; Min, B.G.; Jeong, Y.G.; Lee, S.C.; Jang, J.H.; Koo, G.H. Superhydrophobicity of cotton fabrics treated with silica nanoparticles and water-repellent agent. J. Colloid Interface Sci. 2009, 337, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Sol–gel treatments on cotton fabrics for improving thermal and flame stability: Effect of the structure of the alkoxysilane precursor. Carbohydr. Polym. 2012, 87, 627–635. [Google Scholar] [CrossRef]
- Alongi, J.; Malucelli, G. State of the art and perspectives on sol–gel derived hybrid architectures for flame retardancy of textiles. J. Mater. Chem. 2012, 22, 21805–21809. [Google Scholar] [CrossRef]
- Gao, T.; Maya-Visuet, E.; He, Z.; Castaneda-Lopez, H.; Zvonkina, I.J.; Soucek, M.D. Effect of pigmentation on polyurethane/polysiloxane hybrid coatings. J. Appl. Polym. Sci. 2016, 133, 5. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some comments on the main fire retardancy mechanisms in polymer nanocomposites. Polym. Adv. Technol. 2006, 17, 772–777. [Google Scholar] [CrossRef]







| Chemical Formulae | Name | Code |
|---|---|---|
 | Trimethoxymethylsilane | (TMEOS) |
 | (3-Aminopropyl)trimethoxysilane | (APTMS) |
 | Tetraethylorthosilicate | (TEOS) |
| Sample Code | Precursor | TiO2 NPs Content wt % | P-Based Compound Content wt % ** |
|---|---|---|---|
| CERTi4 | TMEOS | 2.3 | 0 |
| CERTi5 | TMEOS | 0.8 | 0 |
| CERTi7 | APTMS * | 2.3 | 0 |
| CERTi8 | TMEOS | 1.2 | 20 |
| CERTi10 | TEOS | 1.2 | 20 |
| dDLS (nm) | PdI | ζ-potELS (mV) | |
|---|---|---|---|
| CERTi4 | 1222 ± 138 | 0.2 | −19.8 ± 0.5 |
| CERTi5 | 6932 ± 790 | 0.9 | −14.7 ± 0.2 |
| CERTi7 | 73 ± 0.5 | 0.4 | +34.6 ± 2.2 |
| CERTi8 | 1140 ± 59 | 0.6 | −19.9 ± 0.53 |
| CERTi10 | 1496 ± 187 | 0.8 | −6.0 ± 0.09 |
| WCA (°) | Residue% * | Weight Loss% * | |
|---|---|---|---|
| Uncoated | 124.06 ± 2.63 | <1 | >99 |
| CERTi4 | 129.01 ± 8.11 | 18.33 | 81.67 |
| CERTi5 | 130.30 ± 14.01 | 1.50 | 98.79 |
| CERTi7 | 85.17 ± 5.45 | 1.35 | 98.50 |
| CERTi8 | 150.95 ± 24.31 | 31.87 | 68.13 |
| CERTi10 | 137.17 ± 10.81 | 15.63 | 84.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortelli, S.; Costa, A.L. Insulating Thermal and Water-Resistant Hybrid Coating for Fabrics. Coatings 2020, 10, 72. https://doi.org/10.3390/coatings10010072
Ortelli S, Costa AL. Insulating Thermal and Water-Resistant Hybrid Coating for Fabrics. Coatings. 2020; 10(1):72. https://doi.org/10.3390/coatings10010072
Chicago/Turabian StyleOrtelli, Simona, and Anna Luisa Costa. 2020. "Insulating Thermal and Water-Resistant Hybrid Coating for Fabrics" Coatings 10, no. 1: 72. https://doi.org/10.3390/coatings10010072
APA StyleOrtelli, S., & Costa, A. L. (2020). Insulating Thermal and Water-Resistant Hybrid Coating for Fabrics. Coatings, 10(1), 72. https://doi.org/10.3390/coatings10010072