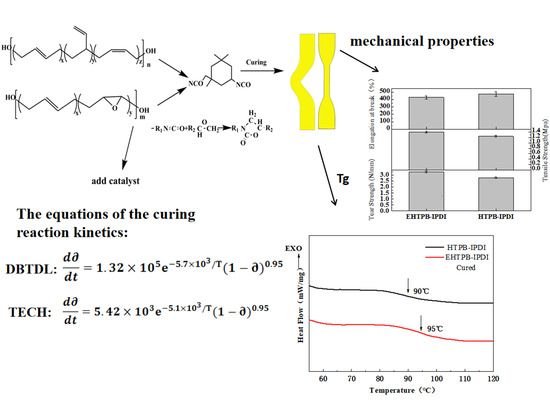
Curing Reaction Kinetics of the EHTPB-Based PBX Binder System and Its Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Calculation of the Curing Reaction Kinetics
2.4. DSC Testing
2.5. Mechanical Properties of EHTPB-IPDI and HTPB-IPDI Polyurethane Elastomers
2.6. Viscosity Monitoring Experiment
3. Results and Discussion
3.1. Determination of the Activation Energy of EHTPB-IPDI and HTPB-IBDI Binder Systems
3.2. Analysis of the Glass Transition Temperature, Tg
3.3. Determination of Mechanical Properties
3.4. Calculation of the Average Activation Energy of the EHTPB-IPDI-DBTDL and EHTPB-IPDI-TECH Binder Systems
3.4.1. Kissinger Method
3.4.2. F-W-O Method
3.4.3. Doyle Method
3.4.4. Average Activation Energy
3.5. Monitoring of Viscosity
3.6. Curing Reaction Kinetic Equations of the EHTPB-IPDI-DBTDL and EHTPB-IPDI-TECH Binder Systems
3.7. Parameters Optimization in the Curing Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krishnan, S.; Swami, R.D. Effect of Burning Rate Modifiers on Subatmospheric Flame Temperatures of AP/HTPB Composite Solid Propellants. Def. Sci. J. 2003, 48, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, A.E.D.M.; Leeuwenburgh, A.B. HNF/HTPB propellants: Influence of HNF particlesize on ballistic properties. Combust. Flame 2009, 156, 1359–1364. [Google Scholar] [CrossRef]
- Chan, M.L.; Reed, R.; Ciaramitaro, D.A. Advances in soild propellant formulations. Prog. Astronaut. Aeronaut. 2000, 185, 185–206. [Google Scholar]
- Jaw, K.-S.; Lee, J.-S. Thermal behaviors of petn base polymer bonded explosives. J. Therm. Anal. Calorim. 2008, 93, 953–957. [Google Scholar] [CrossRef]
- Jia-Hu, G.; Yu-Cun, L.; Tao, C. A sutdy on the HTPB-isocyanate Binder system curing at room temperature and related properties. Explos. Mater. 2015, 44, 7–11. [Google Scholar] [CrossRef]
- Kébir, N.; Campistron, I.; Laguerre, A.; Pilard, J.-F.; Bunel, C.; Couvercelle, J.-P.; Gondard, C. Use of hydroxytelechelic cis-1,4-polyisoprene (HTPI) in the synthesis of polyurethanes (PUs). Part 1. Influence of molecular weight and chemical modification of HTPI on the mechanical and thermal properties of PUs. Polymer 2005, 46, 6869–6877. [Google Scholar] [CrossRef]
- Natarajan, M.; Murugavel, S.C. Thermal stability and thermal degradation kinetics of bio-based epoxy resins derived from cardanol by thermogravimetric analysis. Polym. Bull. 2016, 74, 3319–3340. [Google Scholar] [CrossRef]
- Fu, X.; Fan, X.; Meng, L.; Yu, H.; Liu, X. Synthesis of epoxidized hydroxyl-terminated polybutadiene and its application prospect in propellant. Chem. Propellants Polym. Mater. 2014, 12, 23–26, 31. [Google Scholar]
- Bagheri, R.; Marouf, B.T.; Pearson, R.A. Rubber-toughened epoxies: A critical review. Polym. Rev. 2009, 49, 201–225. [Google Scholar] [CrossRef]
- Lucio, B.; De La Fuente, J.L. Rheokinetic analysis on the formation of metallo-polyurethanes based on hydroxyl-terminated polybutadiene. Eur. Polym. J. 2014, 50, 117–126. [Google Scholar] [CrossRef]
- Catherine, K.B.; Krishnan, K.; Ninan, K.N. A DSC study on cure kinetics of HTPB-IPDI urethane reaction. J. Therm. Anal. Calorim. 2000, 59, 93–100. [Google Scholar] [CrossRef]
- Bina, C.K.; Kannan, K.G.; Ninan, K.N. DSC study on the effect of isocyanates and catalysts on the HTPB cure reaction. J. Therm. Anal. Calorim. 2004, 78, 753–760. [Google Scholar] [CrossRef]
- Hailu, K.; Guthausen, G.; Becker, W.; König, A.; Bendfeld, A.; Geissler, E. In-situ characterization of the cure reaction of HTPB and IPDI by simultaneous NMR and IR measurements. Polym. Test. 2010, 29, 513–519. [Google Scholar] [CrossRef]
- Yang, P.; Yu, Y.H.; Wang, S.P.; Li, T.D. Kinetic studies of isophorone diisocyanate-polyether polymerization with in situ FT-IR. Int. J. Polym. Anal. Charact. 2011, 16, 584–590. [Google Scholar] [CrossRef]
- Haska, S.B.; Bayramli, E.; Pekel, F.; Özkar, S. Mechanical-properties of HTPB-Ipdi-Based elastomers. J. Appl. Polym. Sci. 2015, 64, 2347–2354. [Google Scholar] [CrossRef]
- Toosi, F.S.; Shahidzadeh, M.; Ramezanzadeh, B. An investigation of the effects of pre-polymer functionality on the curing behavior and mechanical properties of HTPB-based polyurethane. J. Ind. Eng. Chem. 2015, 24, 166–173. [Google Scholar] [CrossRef]
- Kincal, D.; Saim, Ö. Kinetic study of the reaction between hydroxyl-terminated polybutadiene and isophorone diisocyanate in bulk by quantitative FTIR spectroscopy. J. Appl. Polym. Sci. 2015, 66, 1979–1983. [Google Scholar] [CrossRef]
- Ma, H.; Yu-Cun, L.; Tao, C.; Tuo-Ping, H.; Jia-Hu, G.; Yan-Wu, Y.; Jun-Ming, Y.; Jian-Hua, W.; Ning, Q.; Liang, Z. Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry. e-Polymers 2017, 17, 89–94. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Y.; Guodong, W.; Chai, T.; Yu, Y.; Yuan, J.; Jing, S.; Feng, F.; Zhong, L.; Zhou, Y.; et al. Catalyzed HTPB/HDI-Trimer Curing Reactions and Influence on Pot Life. Coatings 2020, 10, 1073. [Google Scholar] [CrossRef]
- Guodong, W.; Chai, T.; Liu, Y.; Cui, J.; Ma, H.; Jing, S.; Zhong, L.; Qin, S.; Wang, G.; Ren, X. Kinetic Research on the Curing Reaction of Hydroxyl-Terminated Polybutadiene Based Polyurethane Binder System via FT-IR Measurements. Coatings 2018, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.H.; Lu, L.L.; Tang, Y.W. An Introduction to Thermal Analysis; Science Press: Beijing, China, 2012. [Google Scholar]
- Huang, M.; Lv, S.; Zhou, C. Thermal decomposition kinetics of glycine in nitrogen atmosphere. Thermochim. Acta 2013, 552, 60–64. [Google Scholar] [CrossRef]
- Koga, N. Ozawa’s kinetic method for analyzing thermoanalytical curves. J. Therm. Anal. Calorim. 2013, 113, 1527–1541. [Google Scholar] [CrossRef]
- Chinese Standard: GB/T 528-1998, Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress–Strain Properties. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT528-1998 (accessed on 20 December 2020).
- Chinese Standard: GB/T 529-2008, Rubber, Vulcanized or Thermoplastic—Determination of Tear Strength. Available online: https://www.chinesestandard.net/PDF.aspx/GBT529-2008 (accessed on 20 December 2020).
- Cao, Z.; Jie, S.Y.; Li, B.G. Preparation and properties of epoxided hydroxyl-terminated polybutadiene based polyurethane elastomers. Acta Polym. Sin. 2017, 8, 1350–1357. [Google Scholar] [CrossRef]
- Lei, Y.; An-Chang, L. Synthesis and characterization of polyoxazolidone. New Chem. Mater. 2006, 4, 7–8. [Google Scholar] [CrossRef]
- Meng-Meng, S. Synthesis and Properties of Oxazolidinone Epoxy Resin. Master Thesis, Beijing University of Chemical Technology, Beijing, China, 2013. [Google Scholar] [CrossRef]
- Yang, J.; He, X.; Wang, H.; Liu, X.; Lin, P.; Yang, S.-X.; Fu, S. High-toughness, environment-friendly solid epoxy resins: Preparation, mechanical performance, curing behavior, and thermal properties. J. Appl. Polym. Sci. 2020, 137, 48596. [Google Scholar] [CrossRef]
- Shen, L.; Han, T.; Wu, H.; Guo, S.Y. Effect of Peroxide Content and Filler Type on the Properties of EPDM Rubber. Polym. Mater. Sci. Eng. 2016, 32, 64–68, 74. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.H.; Hong, I.-K.; Lee, J.W. Curing behavior of polyurethane as a binder for polymer-bonded explosives. J. Ind. Eng. Chem. 2015, 21, 980–985. [Google Scholar] [CrossRef]
- Liu, J.R.; Luo, Y.J. Curing kinetics of HTPB/TDI/Al system by non-isothermal DSC. Chin. J. Energetic Mater. 2009, 17, 83–86. [Google Scholar] [CrossRef]
- Daniel, M.A. Polyurethane Binder Systems for Polymer Bonded Explosives; No. DSTO-GD-0492. Defence Science and Technology Organisation Edinburgh (Australia) Weapons Systems DIV,; 2006; Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=871c1a81b1a02a4185435c5e9b1311ef&site=xueshu_se&hitarticle=1 (accessed on 18 December 2020).
- Sekkar, V.; Raunija, T.S.K. Issues Related with Pot Life Extension for Hydroxyl-Terminated Polybutadiene-Based Solid Propellant Binder System. Propellants Explos. Pyrotech. 2015, 40, 267–274. [Google Scholar] [CrossRef]
















| β/°C·min−1 | T1i/°C | T1p/°C | T1f/°C | T2i/°C | T2p/°C | T2f/°C |
|---|---|---|---|---|---|---|
| 5 | 167.4 | 190.4 | 212.7 | 174.5 | 200.1 | 223.3 |
| 10 | 174.8 | 207.8 | 239.6 | 189.5 | 221.1 | 247.4 |
| 15 | 181.4 | 222.4 | 255.6 | 196.4 | 232.7 | 263.1 |
| 20 | 189.1 | 234.2 | 270.2 | 197.7 | 241.9 | 277.4 |
| System | E/kJ·mol−1 | A/s−1 |
|---|---|---|
| EHTPB-IPDI | 53.8 | 1.79 × 105 |
| HTPB-IPDI | 59.1 | 5.29 × 105 |
| System | Tensile Strength (mPa) | Elongations at Break (%) | Tear Strength (N/mm) |
|---|---|---|---|
| HTPB-IPDI | 1.25 ± 0.05 | 473 ± 23 | 2.78 ± 0.07 |
| EHTPB-IPDI | 1.40 ± 0.03 | 429 ± 18 | 3.27 ± 0.05 |
| β/°C·min−1 | T1i/°C | T1p/°C | T1f/°C | T2i/°C | T2p/°C | T2f/°C |
|---|---|---|---|---|---|---|
| 5 | 159.5 | 199.5 | 216.9 | 164.2 | 178.2 | 199.0 |
| 10 | 169.1 | 220.9 | 241.7 | 174.7 | 204.9 | 233.8 |
| 15 | 180.9 | 242.0 | 265.8 | 181.1 | 220.7 | 251.8 |
| 20 | 182.7 | 250.8 | 279.0 | 187.1 | 232.8 | 264.4 |
| Binder System | Ea (kJ/mol) | Correlation Coefficient R2 | ||||
|---|---|---|---|---|---|---|
| - | Kissinger | F-W-O | Doyle | Kissinger | F-W-O | Doyle |
| HTPB-IPDI-TECH | 45.4 | 51.0 | 44.9 | 0.9808 | 0.9865 | 0.9865 |
| EHTPB-IPDI-DBTDL | 40.3 | 45.8 | 40.8 | 0.9994 | 0.9995 | 0.9995 |
| System | Initial Temperature (°C) | Peak Temperature (°C) | Final Temperature (°C) |
|---|---|---|---|
| EHTPB-IPDI-TECH | 153 | 185 | 198 |
| EHTPB-IPDI-DBTDL | 158 | 164 | 184 |
| System | Curing Rate () | Curing Time (t) | Curing Time (h) |
|---|---|---|---|
| EHTPB-IPDI-DBTDL | 4 | ||
| EHTPB-IPDI-TECH | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Y.; Chai, T.; Ma, Z.; Jia, K. Curing Reaction Kinetics of the EHTPB-Based PBX Binder System and Its Mechanical Properties. Coatings 2020, 10, 1266. https://doi.org/10.3390/coatings10121266
Zhang X, Liu Y, Chai T, Ma Z, Jia K. Curing Reaction Kinetics of the EHTPB-Based PBX Binder System and Its Mechanical Properties. Coatings. 2020; 10(12):1266. https://doi.org/10.3390/coatings10121266
Chicago/Turabian StyleZhang, Xing, Yucun Liu, Tao Chai, Zhongliang Ma, and Kanghui Jia. 2020. "Curing Reaction Kinetics of the EHTPB-Based PBX Binder System and Its Mechanical Properties" Coatings 10, no. 12: 1266. https://doi.org/10.3390/coatings10121266
APA StyleZhang, X., Liu, Y., Chai, T., Ma, Z., & Jia, K. (2020). Curing Reaction Kinetics of the EHTPB-Based PBX Binder System and Its Mechanical Properties. Coatings, 10(12), 1266. https://doi.org/10.3390/coatings10121266