Iron-Doped Titanium Dioxide Nanoparticles As Potential Scaffold for Hydrazine Chemical Sensor Applications
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis of Fe-Doped TiO2 Nanoparticles
2.2. Characterizations of Fe-Doped TiO2 Nanoparticles
2.3. Hydrazine Chemical Sensor Fabrication
3. Results and Discussion
3.1. Characterizations and Properties of Fe-Doped TiO2 Nanoparticles
3.2. Electrochemical Sensing Properties of Hydrazine Using Fe-Doped TiO2 Nanoparticles
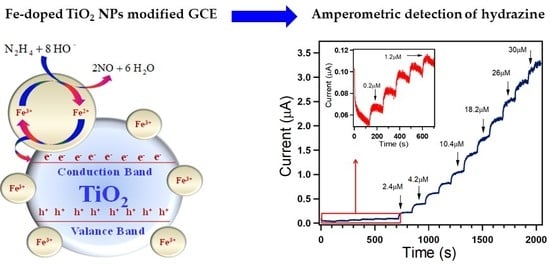
3.3. Amperometric Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wahab, R.; Ahmad, N.; Alam, M.; Ahmed, J. Nanorods of ZnO: An effective hydrazine sensor and their chemical properties. Vacuum 2019, 165, 290–296. [Google Scholar] [CrossRef]
- Guo, S.-H.; Guo, Z.-Q.; Wang, C.-Y.; Shen, Y.; Zhu, W.-H. An ultrasensitive fluorescent probe for hydrazine detection and its application in water samples and living cells. Tetrahedron 2019, 75, 2642–2646. [Google Scholar] [CrossRef]
- Nemakal, M.; Aralekallu, S.; Mohammed, I.; Swamy, S.; Sannegowda, L.K. Electropolymerized octabenzimidazole phthalocyanine as an amperometric sensor for hydrazine. J. Electroanal. Chem. 2019, 839, 238–246. [Google Scholar] [CrossRef]
- Wang, L.; Meng, T.; Jia, H.; Feng, Y.; Gong, T.; Wang, H.; Zhang, Y. Electrochemical study of hydrazine oxidation by leaf-shaped copper oxide loaded on highly ordered mesoporous carbon composite. J. Colloid Interface Sci. 2019, 549, 98–104. [Google Scholar] [CrossRef]
- Duan, C.; Dong, Y.; Sheng, Q.; Zheng, J. A high-performance non-enzymatic electrochemical hydrazine sensor based on NiCo2S4 porous sphere. Talanta 2019, 198, 23–29. [Google Scholar] [CrossRef]
- George, M.; Nagaraja, K.S.; Balasubramanian, N. Spectrophotometric determination of hydrazine. Talanta 2008, 75, 27–31. [Google Scholar] [CrossRef]
- Rios, A.; Silva, M.; Valcarcel, M. Fluorimetric determination of ammonia, hydrazine and hydroxylamine and their mixtures by differential kinetic methods. Fresenius’ Zeitschrift Für Anal. Chem. 1985, 320, 762–768. [Google Scholar] [CrossRef]
- Collins, G.E.; Rose-Pehrsson, S.L. Sensitive, fluorescent detection of hydrazine via derivatization with 2,3-naphthalene dicarboxaldehyde. Anal. Chim. Acta 1993, 284, 207–215. [Google Scholar] [CrossRef]
- Collins, G.E.; Latturner, S.; Rose-Pehrsson, S.L. Chemiluminescence detection of hydrazine vapor. Talanta 1995, 42, 543–551. [Google Scholar] [CrossRef]
- Bravo, P.; Isaacs, F.; Ramírez, G.; Azócar, I.; Trollund, E.; Aguirre, M.J. A potentiometric hydrazine sensor: Para-Ni-tetraaminophenylporphyrin/Co-cobaltite/SNO2:F modified electrode. J. Coord. Chem. 2007, 60, 2499–2507. [Google Scholar] [CrossRef]
- Ganesh, S.; Khan, F.; Ahmed, M.K.; Pandey, S.K. Potentiometric determination of free acidity in presence of hydrolysable ions and a sequential determination of hydrazine. Talanta 2011, 85, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.K.; Moshkalev, S.A.; Rout, C.S. Electrochemical sensing of hydrazine using multilayer graphene nanobelts. RSC Adv. 2016, 6, 11329–11334. [Google Scholar] [CrossRef] [Green Version]
- Rostami, S.; Azizi, S.N.; Ghasemi, S. Simultaneous electrochemical determination of hydrazine and hydroxylamine by CuO doped in ZSM-5 nanoparticles as a new amperometric sensor. New J. Chem. 2017, 41, 13712–13723. [Google Scholar] [CrossRef]
- Jena, B.K.; Raj, C.R. Ultrasensitive nanostructured platform for the electrochemical sensing of hydrazine. J. Phys. Chem. C 2007, 111, 6228–6232. [Google Scholar] [CrossRef]
- Deng, J.; Deng, S.; Liu, Y. Highly sensitive electrochemical sensing platform for hydrazine detection. Int. J. Electrochem. Sci. 2018, 13, 3566–3574. [Google Scholar] [CrossRef]
- Gu, X.; Li, X.; Wu, S.; Shi, J.; Jiang, G.; Jiang, G.; Tian, S. A sensitive hydrazine hydrate sensor based on a mercaptomethyl-terminated trinuclear Ni(ii) complex modified gold electrode. RSC Adv. 2016, 6, 8070–8078. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Yang, S.; Tian, H.; Liu, Y.; Hao, Y.; Zhang, J.; Sun, B. A Fluorescent Probe for Sensitive Detection of Hydrazine and Its Application in Red Wine and Water. Anal. Sci. 2018, 34, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.J. Electrodeposition of ZnO Nanofibers for Sensitive Determination of Hydrazine and Nitrite. Sens. Lett. 2017, 14, 1187–1192. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Wang, R.; Wang, H.; Lv, W.; Ji, S. Ultrathin willow-like CuO nanoflakes as an efficient catalyst for electro-oxidation of hydrazine. J. Power Sources 2015, 289, 22–25. [Google Scholar] [CrossRef]
- Wang, G.; Gu, A.; Wang, W.; Wei, Y.; Wu, J.; Wang, G.; Zhang, X.; Fang, B. Copper oxide nanoarray based on the substrate of Cu applied for the chemical sensor of hydrazine detection. Electrochem. Commun. 2009, 11, 631–634. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, C.; He, X.; Li, Z.; Zhang, X.; Wang, L.; Fang, B. Detection of hydrazine based on Nano-Au deposited on Porous-TiO2 film. Electrochim. Acta 2010, 55, 7204–7210. [Google Scholar] [CrossRef]
- Yi, Q.; Niu, F.; Yu, W. Pd-modified TiO2 electrode for electrochemical oxidation of hydrazine, formaldehyde and glucose. Thin Solid Films 2011, 519, 3155–3161. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, T.; Wang, Q.; Umar, A. Morphology and chemical composition dependent synthesis and electrochemical properties of MnO2-based nanostructures for efficient hydrazine detection. Sens. Actuators B Chem. 2016, 224, 878–884. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Pinjari, D.V.; Gogate, P.R.; Sonawane, S.H.; Pandit, A.B. Ultrasound assisted synthesis of doped TiO 2 nano-particles: Characterization and comparison of effectiveness for photocatalytic oxidation of dyestuff effluent. Ultrason. Sonochem. 2013, 20, 277–286. [Google Scholar] [CrossRef]
- Khodari, M.; Mersal, G.A.M.; Rabie, E.M.; Assaf, H.F. Electrochemical sensor based on carbon paste electrode modified by TiO2 nano-particles for the voltammetric determination of resorcinol. Int. J. Electrochem. Sci. 2018, 13, 3460–3474. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, M.; Li, X.; Li, X.; Li, H.; Lu, Y.; Song, X.; Wang, L. A novel CuO/TiO 2 hollow nanofiber film for non-enzymatic glucose sensing. RSC Adv. 2016, 6, 99969–99976. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Zhang, M.; Hou, H.; Song, Y.; Wang, H.; Zhong, B.; Wang, L. Hierarchically mesostructured porous TiO2 hollow nanofibers for high performance glucose biosensing. Biosens. Bioelectron. 2017, 92, 654–660. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, K.; Lv, F.; Huang, H.; Fei, B.; He, Y.; Ye, Z.; Shen, B. Photocatalytic treatment of 2,4,6-trinitotoluene in red water by multi-doped TiO2 with enhanced visible light photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 452, 103–108. [Google Scholar] [CrossRef]
- Bouazizi, N.; Bargougui, R.; Boudharaa, T.; Khelil, M.; Benghnia, A.; Labiadh, L.; Slama, R.B.; Chaouachi, B.; Ammar, S.; Azzouz, A. Synthesis and characterization of SnO2-HMD-Fe materials with improved electric properties and affinity towards hydrogen. Ceram. Int. 2016, 42, 9413–9418. [Google Scholar] [CrossRef]
- Bouazizi, N.; Ajala, F.; Khelil, M.; Lachheb, H.; Khirouni, K.; Houas, A.; Azzouz, A. Zinc oxide incorporating iron nanoparticles with improved conductance and capacitance properties. J. Mater. Sci. Mater. Electron. 2016, 27, 11168–11175. [Google Scholar] [CrossRef]
- Bouazizi, N.; Ajala, F.; Bettaibi, A.; Khelil, M.; Benghnia, A.; Bargougui, R.; Louhichi, S.; Labiadh, L.; Slama, R.B.; Chaouachi, B.; et al. Metal-organo-zinc oxide materials: Investigation on the structural, optical and electrical properties. J. Alloys Compd. 2016, 656, 146–153. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Siwach, R.; Kumar, S.; Alhazaa, A.N. Role of Fe doping in tuning photocatalytic and photoelectrochemical properties of TiO2 for photodegradation of methylene blue. Opt. Laser Technol. 2019, 118, 170–178. [Google Scholar] [CrossRef]
- Moradi, V.; Ahmed, F.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Acid-treated Fe-doped TiO2 as a high performance photocatalyst used for degradation of phenol under visible light irradiation. J. Environ. Sci. China 2019, 83, 183–194. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef]
- Ismael, M. Enhanced photocatalytic hydrogen production and degradation of organic pollutants from Fe (III) doped TiO2 nanoparticles. J. Environ. Chem. Eng. 2020, 8, 103676. [Google Scholar] [CrossRef]
- Khan, M.A.; Woo, S.I.; Yang, O.B. Hydrothermally stabilized Fe(III) doped titania active under visible light for water splitting reaction. Int. J. Hydrogen Energy 2008, 33, 5345–5351. [Google Scholar] [CrossRef]
- Niu, Y.; Xing, M.; Zhang, J.; Tian, B. Visible light activated sulfur and iron co-doped TiO2 photocatalyst for the photocatalytic degradation of phenol. Catal. Today 2013, 201, 159–166. [Google Scholar] [CrossRef]
- Ali, I.; Kim, S.-R.; Park, K.; Kim, J.-O. One-step electrochemical synthesis of graphene oxide-TiO2 nanotubes for improved visible light activity. Opt. Mater. Express 2017, 7, 1535. [Google Scholar] [CrossRef]
- Prajapati, B.; Kumar, S.; Kumar, M.; Chatterjee, S.; Ghosh, A.K. Investigation of the physical properties of Fe:TiO2 -diluted magnetic semiconductor nanoparticles. J. Mater. Chem. C 2017, 5, 4257–4267. [Google Scholar] [CrossRef]
- Dong, B.; He, B.L.; Huang, J.; Gao, G.Y.; Yang, Z.; Li, H.L. High dispersion and electrocatalytic activity of Pd/titanium dioxide nanotubes catalysts for hydrazine oxidation. J. Power Sources 2008, 175, 266–271. [Google Scholar] [CrossRef]
- Shukla, S.; Chaudhary, S.; Umar, A.; Chaudhary, G.R.; Mehta, S.K. Tungsten oxide (WO3) nanoparticles as scaffold for the fabrication of hydrazine chemical sensor. Sens. Actuators B Chem. 2014, 196, 231–237. [Google Scholar] [CrossRef]
- Li, Z.; Shen, W.; He, W.; Zu, X. Effect of Fe-doped TiO2 nanoparticle derived from modified hydrothermal process on the photocatalytic degradation performance on methylene blue. J. Hazard. Mater. 2008, 155, 590–594. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, W.; He, B.; Zhang, J.; Anpo, M. Characterization of Fe-TiO2 photocatalysts synthesized by hydrothermal method and their photocatalytic reactivity for photodegradation of XRG dye diluted in water. J. Mol. Catal. A Chem. 2004, 216, 35–43. [Google Scholar] [CrossRef]
- Dong, B.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. Facile Synthesis and High Activity of Novel Ag/TiO2-NTs Composites for Hydrazine Oxidation. Adv. Mater. Res. 2011, 197, 1073–1078. [Google Scholar] [CrossRef]
- Dong, B.; He, B.L.; Chai, Y.M.; Liu, C.G. Novel Pt nanoclusters/titanium dioxide nanotubes composites for hydrazine oxidation. Mater. Chem. Phys. 2010, 120, 404–408. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.N. Statistics and Chemometrics for Analytical Chemistry, 5th ed.; Pearson Prentice Hall: Edinburgh, Scotland, 2005. [Google Scholar]
- Faisal, M.; Harraz, F.A.; Al-Salami, A.E.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Polythiophene/ZnO nanocomposite-modified glassy carbon electrode as efficient electrochemical hydrazine sensor. Mater. Chem. Phys. 2018, 214, 126–134. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Z.; Sheng, Q.; Zheng, J. Solvothermal synthesis of Ag@Fe3O4 nanosphere and its application as hydrazine sensor. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 371–377. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Alamry, K.A. Sensitive and selective m-tolyl hydrazine chemical sensor development based on CdO nanomaterial decorated multi-walled carbon nanotubes. J. Ind. Eng. Chem. 2019, 77, 309–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, B.; Ye, J.; Ye, J. A sensitive and selective amperometric hydrazine sensor based on palladium nanoparticles loaded on cobalt-wrapped nitrogen-doped carbon nanotubes. J. Electroanal. Chem. 2017, 801, 215–223. [Google Scholar] [CrossRef]
- Gioia, D.; Casella, I.G. Pulsed electrodeposition of palladium nano-particles on coated multi-walled carbon nanotubes/nafion composite substrates: Electrocatalytic oxidation of hydrazine and propranolol in acid conditions. Sens. Actuators B Chem. 2016, 237, 400–407. [Google Scholar] [CrossRef]
- Tang, Y.-Y.; Kao, C.-L.; Chen, P.-Y. Electrochemical detection of hydrazine using a highly sensitive nanoporous gold electrode. Anal. Chim. Acta 2012, 711, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Harraz, F.A.; Ismail, A.A.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Highly sensitive amperometric hydrazine sensor based on novel α-Fe2O3/crosslinked polyaniline nanocomposite modified glassy carbon electrode. Sens. Actuators B Chem. 2016, 234, 573–582. [Google Scholar] [CrossRef]
- Zhou, T.; Lu, P.; Zhang, Z.; Wang, Q.; Umar, A. Perforated Co3O4 nanoneedles assembled in chrysanthemum-like Co3O4 structures for ultra-high sensitive hydrazine chemical sensor. Sens. Actuators B Chem. 2016, 235, 457–465. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Li, P.; Zhang, W.; Lian, K.; Hu, J.; Chen, Y. Preparation and characterization of AuNPs/CNTs-ErGO electrochemical sensors for highly sensitive detection of hydrazine. Talanta 2016, 158, 283–291. [Google Scholar] [CrossRef] [Green Version]







| Sensor Electrode | Sensitivity | LOD | Ref. |
|---|---|---|---|
| Leaf shape CuO/OMC/GCE | 0.00487 μA.μM−1·cm−2 | 0.887 μM | [4] |
| NiCo2S4 sphere/GCE | 179.1μA.mM−1·cm−2 | 0.6μM | [5] |
| WO3 NPs/Au electrode | 0.185 μA.μM−1·cm−2 | 144.73 μM | [32] |
| Polythiophene/ZnO/GCE | 1.22 μA.μM−1·cm−2 | 0.207μM | [48] |
| Ag@Fe3O4 nanosphere/GCE | 270.0μA.mM−1·cm−2 | 0.06μM | [49] |
| CdO/CNT nanocomposites/GCE | 25.79μA.μM−1·cm−2 | 4.0 pM | [50] |
| Pd/Co-NCNTs | 343.9 μA.mM−1·cm−2 | 0.007 μM | [51] |
| Pd (CNT-Pd)/GCE | 0.3 μA.mM−1·cm−2 | 8.0 μM | [52] |
| Nanoporous gold/ITO | 0.161 μA.μM−1·cm−2 | 0.0043 μM | [53] |
| α-Fe2O3/polyaniline nanocomposite | 1.93 mA.μM−1·cm−2 | 0.153 μM | [54] |
| Chrysanthemum-like Co3O4/GCE | 107.9 μA.mM−1·cm−2 | 3.7 μΜ | [55] |
| AuNPs/CNTs-rGO/GCE | 9.73 μA.μM−1·cm−2 | 0.065 μΜ | [56] |
| Fe-doped TiO2 NPs/GCE | 1.44 μA.µM−1·cm−2 | 0.236 μΜ | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, A.; Harraz, F.A.; Ibrahim, A.A.; Almas, T.; Kumar, R.; Al-Assiri, M.S.; Baskoutas, S. Iron-Doped Titanium Dioxide Nanoparticles As Potential Scaffold for Hydrazine Chemical Sensor Applications. Coatings 2020, 10, 182. https://doi.org/10.3390/coatings10020182
Umar A, Harraz FA, Ibrahim AA, Almas T, Kumar R, Al-Assiri MS, Baskoutas S. Iron-Doped Titanium Dioxide Nanoparticles As Potential Scaffold for Hydrazine Chemical Sensor Applications. Coatings. 2020; 10(2):182. https://doi.org/10.3390/coatings10020182
Chicago/Turabian StyleUmar, Ahmad, Farid A. Harraz, Ahmed A. Ibrahim, Tubia Almas, Rajesh Kumar, M. S. Al-Assiri, and Sotirios Baskoutas. 2020. "Iron-Doped Titanium Dioxide Nanoparticles As Potential Scaffold for Hydrazine Chemical Sensor Applications" Coatings 10, no. 2: 182. https://doi.org/10.3390/coatings10020182
APA StyleUmar, A., Harraz, F. A., Ibrahim, A. A., Almas, T., Kumar, R., Al-Assiri, M. S., & Baskoutas, S. (2020). Iron-Doped Titanium Dioxide Nanoparticles As Potential Scaffold for Hydrazine Chemical Sensor Applications. Coatings, 10(2), 182. https://doi.org/10.3390/coatings10020182