Cross-Linking of Thermally Hydrolyzed Specified Risk Materials with Epoxidized Poly (Vinyl Alcohol) for Tackifier Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2.1. SRM Hydrolysis and Recovery
2.2.2. Epoxidation of PVA
2.2.3. Epoxy Content Determination
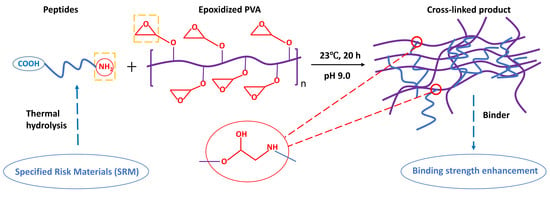
2.2.4. Cross-Linking of SRM-Derived Peptides with Epoxidized PVA
2.2.5. Primary Amino Groups Characterization
2.2.6. Carboxylic Groups Characterization
2.2.7. Compression Strength Analysis
2.2.8. Thermogravimetric Analysis (TGA)
2.2.9. Moisture-Holding Capacity
2.2.10. Aqueous Viscosity Analysis
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Cross-Linking of SRM-Derived Peptides with Unmodified PVA
3.2. Synthesis and Characterization of Epoxidized PVA
3.2.1. Impact of NaOH Concentration
3.2.2. Impact of Reaction Temperature
3.2.3. Impact of Reaction Time
3.2.4. Impact of Molar Ratio
3.2.5. Summary of PVA Epoxidation
3.3. Synthesis of PEP Composites
3.3.1. Assessing the Degree of Cross-Linking through Primary Amine Group Quantification
Role of Cross-Linking Temperature
Role of Cross-Linking Time
3.3.2. Assessing the Degree of Cross-Linking through Carboxylic Acid Group Quantification
3.4. Application of PEP Products as A Tackifiers
3.4.1. Compression Strength of Pucks Produced Using PEP
3.4.2. Thermal Resistance of PEP
3.4.3. Moisture-Holding Capacity of PEP
3.4.4. Aqueous Viscosity of PEP
3.4.5. Estimated Cost of Tackifiers Derived from Peptides
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Mekonnen, T.H.; Paolo, M.; El-Thaher, N.; Choi, P.Y.K.; Bressler, D.C. Thermosetting proteinaceous plastics hydrolyzed specified risk material. Macromol. Mater. Eng. 2009, 298, 1294–1303. [Google Scholar] [CrossRef]
- Mekonnen, T.H. Valorization of Waste Protein Biomass for Bio-Based Plastics, Composites and Adhesives Development. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2014. [Google Scholar]
- Canadian Food Inspection Agency. Enhanced Feed Ban Decision Documents. Available online: https://www.inspection.gc.ca/animal-health/terrestrial-animals/diseases/reportable/bse/enhanced-feed-ban/eng/1424374475489/1424374476208 (accessed on 1 March 2020).
- Mekonnen, T.H.; Mussone, P.G.; Stashko, N.; Choi, P.Y.K.; Bressler, D.C. Recovery and characterization of proteinaceous material recovered from thermal and alkaline hydrolyzed specified risk materials. Process. Biochem. 2013, 48, 885–892. [Google Scholar] [CrossRef]
- Mekonnen, T.H.; Mussone, P.G.; El-Thaher, N.; Choi, P.Y.K.; Bressler, D.C. Subcritical hydrolysis and characterization of waste proteinaceous biomass for value added applications. J. Chem. Technol. Biotechnol. 2015, 90, 476–483. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency. Review of Enhanced Feed Ban. 2012. Available online: https://www.inspection.gc.ca/animal-health/terrestrial-animals/diseases/reportable/bse/srm/2012-review/eng/1395765714996/1395766363717 (accessed on 1 March 2020).
- Shui, T.; Adhikari, B.B.; Chae, M.; Bressler, D.C. Evaluation of thermally hydrolyzed specified risk materials cross-linked with glutaraldehyde for tackifier applications. Process Org. Coat. 2020, 140, 105535. [Google Scholar] [CrossRef]
- Alberta Prion Research Institute International Discussion on Specified Risk Materials. Canmore, Alberta, 2015. Available online: http://prioninstitute.ca/admin/ckfinder/userfiles/files/SRM_Report.pdf (accessed on 22 June 2020).
- Krapas, D.; Beacham, J.; Bell, J.; Borgias, A.P.; Donovan, J.; Iwasaki, B.; Lahti, G.; Schmidt, L.; Williams, E. Hydroseed Pilot Project Summary Report. Spokane River Regional Toxics Task Force. Available online: http://srrttf.org/wp-content/uploads/2015/03/Hydroseed-Pilot-Project-Report-FINAL.pdf (accessed on 1 March 2020).
- Parsakhoo, A.; Jajouzadeh, M.; Motlagh, A.R. Effect of hydroseeding on grass yield and water use efficiency on forest road artificial soil slopes. J. For. Sci. 2018, 64, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Brzozowski, C. Hydroseeding strategies: Experts discuss what they use in the mix and how to apply it. Eros. Control 2006, 13, 60–71. [Google Scholar]
- Tackifiers. Soil Erosion and Hydroseeding. Available online: http://soilerosiononline.com/article-permalink-450.html (accessed on 1 March 2020).
- Goldberg, S. Tough hydroseeding challenges: Establishing vegetation on steep slopes and at high altitudes. Eros. Control 2009, 16, 18–27. [Google Scholar]
- Anon. Starch Wholesale Price Quote. Available online: http://www.cooperativepurchasers.com/Ingredients/Starches/,2012b (accessed on 1 March 2020).
- Greg, T.H. Bioconjugate Techniques, 3rd ed.; Chapter 5; Academic Press: Cambridge, MA, USA, 2013; pp. 294–295. [Google Scholar]
- Shechter, L.; Wynstra, J.; Raymond, P.K. Glycidyl ether reactions with amines. Ind. Eng. Chem. 1956, 48, 94–97. [Google Scholar] [CrossRef]
- Motawie, A.M.; Sherif, M.H.; Badr, M.M.; Amer, A.A.; Shehat, A.S. Synthesis and characterization of waterborne epoxy resins for coating application. Australian J. Basic Appl. Sci. 2010, 4, 1376–1382. [Google Scholar]
- Li, M.; Tao, W.; Lu, S.; Kuga, S. Compliant film of regenerated Antheraea pernyi silk fibroin by chemical crosslinking. Int. J. Biol. Macromol. 2003, 32, 159–163. [Google Scholar] [CrossRef]
- Titova, E.F.; Obolonkova, E.S.; Belavtseva, E.M. Electron microscopic study of solutions of dextran and polyvinyl alcohol. Farmatsiia 1974, 23, 12. [Google Scholar]
- Alupei, I.C.; Popa, M.; Hamcerencu, M.; Abadie, M.J.M. Superabsorbent hydrogels based on xanthan and poly (vinyl alcohol) 1. The study of the swelling properties. Eur. Polym. J. 2002, 38, 2313–2320. [Google Scholar] [CrossRef]
- Birendra, B.A.; Chae, M.; Zhu, C.; Khan, A.; Harfield, D.; Choi, P.; Bressler, D.C. Pelletization of torrefied wood using a proteinaceous binder developed from hydrolyzed specified risk materials. Processes 2019, 7, 229. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Wang, Y.; Zhao, T.; Ye, Z.; Huang, H. Ultrasonication-assisted rapid determination of epoxide value in polymer mixtures containing epoxy resin. Anal. Methods 2014, 6, 4257–4261. [Google Scholar]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Part No. 20036; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Vaz, C.M.; De Graaf, L.A.; Reis, R.L.; Cunha, A.M. In vitro degradation behaviour of biodegradable soy plastics: Effects of crosslinking with glyoxal and thermal treatment. Polym. Degrad. Stab. 2003, 81, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Morrison, S.; Sosnoff, J.J.; Heffernan, K.S.; Jae, S.Y.; Fernhall, B. Ageing, hypertension and physiological tremor: The contribution of the cardioballistic impulse to tumorigenesis in older adults. J. Neurol. Sci. 2013, 326, 68–74. [Google Scholar] [CrossRef]
- Veronese, F.M. Peptide and protein PEGylation: A review of problems and solutions. Biomaterials 2001, 22, 405–417. [Google Scholar] [CrossRef]
- Haweel, C.K.; Ammar, S.H. Preparation of polyvinyl alcohol from local raw materials. J. Chem. Pet. Eng. 2008, 9, 15–21. [Google Scholar]
- Garnica-Palafox, I.M.; Sánchez-Arévalo, F.M.; Velasquillo, C.; García-Carvajal, Z.Y.; García-López, J.; Ortega-Sánchez, C.; Ibarra, C.; Luna-Bárcenas, G.; Solís-Arrieta, L. Mechanical and structural response of a hybrid hydrogel based on chitosan and poly(vinyl alcohol) cross-linked with epichlorohydrin for potential use in tissue engineering. J. Biomat. Sci. Polym. Ed. 2014, 25, 32–50. [Google Scholar] [CrossRef]
- Kim, D.H.; Na, S.K.; Park, J.S.; Yoon, K.J.; Ihm, D.W. Studies on the preparation of hydrolyzed starch-g-PAN (HSPAN)/PVA blend films—Effect of the reaction with epichlorohydrin. Eur. Polym. J. 2002, 38, 1199–1204. [Google Scholar] [CrossRef]
- Chang, C.; Lue, A.; Zhang, L. Effects of crosslinking methods on structure and properties of cellulose/PVA hydrogels. Macromol. Chem. Phys. 2008, 209, 1266–1273. [Google Scholar] [CrossRef]
- Shechter, L.; Wynstra, J. Glycidyl Ether reactions with alcohols, phenols, carboxylic acids and acid anhydrides. Ind. Eng. Chem. 1956, 48, 86–93. [Google Scholar] [CrossRef]
- QDOCEAN Import and Export Co. Available online: https://www.alibaba.com/product-detail/Hot-selling-high-quality-epichlorohydrinwith_62301919358.html?spm=a2700.galleryofferlist.0.0.1458d6baN6z5oW (accessed on 1 March 2020).
- Guangzhou Minwei PVA Sales Co. Polyvinyl Alcohol. Polyvinyl Alcohol. Available online: https://www.alibaba.com/product-detail/Polyvinyl-Alcohol-PVA-PVOH-Flakes-Sinopec_60699356572.html?spm=a2700.galleryofferlist.0.0.2bc355e7nIvkPU (accessed on 1 March 2020).









| PVA (g) | No. of Hydroxyl Groups (mmol) | Epichlorohydrin (mL) | No. of Epoxy Groups (mmol) | Molar Ratio of Hydroxyl Groups: Epoxy Groups |
|---|---|---|---|---|
| 1.14 | 25.8 | 1.0 | 12.9 | 1:0.5 |
| 1.14 | 25.8 | 2.0 | 25.8 | 1:1 |
| 1.14 | 25.8 | 4.0 | 51.6 | 1:2 |
| 1.14 | 25.8 | 6.0 | 77.4 | 1:3 |
| 1.14 | 25.8 | 8.0 | 103.1 | 1:4 |
| Epoxidized PVA (mL) | No. of Epoxy Groups (mmol) | Mass of the SRM-Derived Peptides (g) | Molar Ratio of Epoxy Groups: Primary Amino Groups |
|---|---|---|---|
| 15 | 2.33 | 8.00 | 0.5:1 |
| 15 | 2.33 | 4.00 | 1:1 |
| 15 | 2.33 | 2.00 | 2:1 |
| 15 | 2.33 | 1.30 | 3:1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shui, T.; Chae, M.; Bressler, D.C. Cross-Linking of Thermally Hydrolyzed Specified Risk Materials with Epoxidized Poly (Vinyl Alcohol) for Tackifier Applications. Coatings 2020, 10, 630. https://doi.org/10.3390/coatings10070630
Shui T, Chae M, Bressler DC. Cross-Linking of Thermally Hydrolyzed Specified Risk Materials with Epoxidized Poly (Vinyl Alcohol) for Tackifier Applications. Coatings. 2020; 10(7):630. https://doi.org/10.3390/coatings10070630
Chicago/Turabian StyleShui, Tao, Michael Chae, and David C. Bressler. 2020. "Cross-Linking of Thermally Hydrolyzed Specified Risk Materials with Epoxidized Poly (Vinyl Alcohol) for Tackifier Applications" Coatings 10, no. 7: 630. https://doi.org/10.3390/coatings10070630
APA StyleShui, T., Chae, M., & Bressler, D. C. (2020). Cross-Linking of Thermally Hydrolyzed Specified Risk Materials with Epoxidized Poly (Vinyl Alcohol) for Tackifier Applications. Coatings, 10(7), 630. https://doi.org/10.3390/coatings10070630