Novel Rapid Protein Coating Technique for Silicon Photonic Biosensor to Improve Surface Morphology and Increase Bioreceptor Density
Abstract
:1. Introduction
2. Differences of APTES-GA, EDC-NHS and the Proposed APTES-(EDC/NHS) Coating Systems
2.1. Conventional APTES-GA and EDC-NHS Coating Techniques
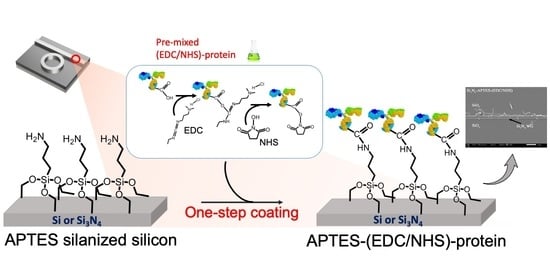
2.2. The Proposed APTES-(EDC/NHS) Coating Technique
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Procedure for Protein Coating
3.2.1. Cleaning and Silanization
3.2.2. APTES-GA Coating Procedure
3.2.3. The Proposed APTES-(EDC/NHS) Coating Procedure
3.3. Measurements of Coating Effectiveness
3.3.1. Surface Morphology Measurements
3.3.2. Surface Element Composition
3.3.3. Bioreaction Measurements
4. Results and Discussions
4.1. Biocoating of Silicon Wafer (Nondevice)
4.2. Surface Element Composition
4.3. Bioreaction Measurement
4.4. Results of Silicon and Silicon Nitride Waveguide Devices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nasiri, S.; Khosravani, M.R. Progress and Challenges in Fabrication of Wearable Sensors for Health Monitoring. Sens. Actuat. A Phys. 2020, 312, 112105. [Google Scholar] [CrossRef]
- Hashem, M.; Al Kheraif, A.A.; Fouad, H. Design and Development of Wireless Wearable Bio-tooth Sensor for Monitoring of Tooth Fracture and Its Bio metabolic Components. Comput. Commun. 2020, 150, 278–285. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A Graphene-based Electrochemical Device with Thermoresponsive Microneedles for Diabetes Monitoring and Therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Shiomi, M.; Fujita, Y.; Arie, T.; Akita, S.; Takei, K. A Wearable pH Sensor with High Sensitivity Based on a Flexible Charge-coupled Device. Nat. Electron. 2018, 1, 596–603. [Google Scholar] [CrossRef]
- Shi, Q.; Dong, B.; He, T.; Sun, Z.; Zhu, J.; Zhang, Z.; Lee, C. Progress in Wearable Electronics/photonics—Moving toward the Era of Artificial Intelligence and Internet of Things. InfoMat 2020, 2, 1131–1162. [Google Scholar] [CrossRef]
- Farandos, N.M.; Yetisen, A.K.; Monteiro, M.J.; Lowe, C.R.; Yun, S.H. Contact Lens Sensors in Ocular Diagnostics. Adv. Healthc. Mater. 2015, 4, 792–810. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Goi, K.; Ishikura, N.; Omichi, K. Silicon Photonics Based System-On-Chip Gas Sensor. In Proceedings of the 26th International Conference on Optical Fiber Sensors, Lausanne, Switzerland, 24–28 September 2018; p. ThE46. [Google Scholar]
- Mi Kyoung, P.; Qing, L.; Kyung Woo, K.; Yong, S.; Jack Sheng, K.; Junfeng, S.; Guo-Qiang, L.; Dim-Lee, K. Integrated Silicon Microring Resonator Devices for Point-of-care Diagnostic Applications. In Proceedings of the SPIE Photonics West, San Francisco, CA, USA, 1–6 February 2014. [Google Scholar]
- Luan, E.; Shoman, H.; Ratner, D.M.; Cheung, K.C.; Chrostowski, L. Silicon Photonic Biosensors Using Label-Free Detection. Sensors 2018, 18, 3519. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, T.; Hirowatari, A.; Ikeda, T.; Fukuyama, M.; Amemiya, Y.; Kuroda, A.; Yokoyama, S. Detection of Antibody-antigen Reaction by Silicon Nitride Slot-ring Biosensors Using Protein G. Opt. Commun. 2016, 365, 16–23. [Google Scholar] [CrossRef]
- Gandolfi, D.; Guider, R.; Chalyan, T.; Pavesi, L.; Pasquardini, L.; Pederzolli, C.; Samusenko, A.; Pucker, G. Sensitivity and Limit of Detection of Biosensors Based on Ring Resonators. In Proceedings of the 2015 XVIII AISEM Annual Conference, Trento, Italy, 3–5 February 2015; pp. 1–4. [Google Scholar]
- Chen, Y.Q.; Yu, F.; Yang, C.; Song, J.Y.; Tang, L.H.; Li, M.Y.; He, J.J. Label-free Biosensing Using Cascaded Double-microring Resonators Integrated with Microfluidic Channels. Opt. Commun. 2015, 344, 129–133. [Google Scholar] [CrossRef]
- Wang, X.; Kim, S.B.; Khang, D.; Kim, H.-H.; Kim, C.-J. Optimization and Characterization of Covalent Immobilization of Glucose Oxidase for Bioelectronic Devices. Biochem. Eng. J. 2016, 112, 20–31. [Google Scholar] [CrossRef]
- Syshchyk, O.; Skryshevsky, V.A.; Soldatkin, O.O.; Soldatkin, A.P. Enzyme Biosensor Systems Based on Porous Silicon Photoluminescence for Detection of Glucose, Urea and Heavy Metals. Biosens. Bioelectron. 2015, 66, 89–94. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Otitoju, T.A.; Ooi, B.S. Optimization of a High Performance 3-aminopropyltriethoxysilane-silica Impregnated Polyethersulfone Membrane Using Response Surface Methodology for Ultrafiltration of Synthetic Oil-water Emulsion. J. Taiwan Inst. Chem. Eng. 2018, 93, 461–476. [Google Scholar] [CrossRef]
- Shim, J.; Park, J.-H. Optimization of Graphene-MoS2 Barristor by 3-aminopropyltriethoxysilane (APTES). Org. Electron. 2016, 33, 172–177. [Google Scholar] [CrossRef]
- Chiadò, A.; Palmara, G.; Ricciardi, S.; Frascella, F.; Castellino, M.; Tortello, M.; Ricciardi, C.; Rivolo, P. Optimization and Characterization of a Homogeneous Carboxylic Surface Functionalization for Silicon-based Biosensing. Colloids Surf. B Biointerfaces 2016, 143, 252–259. [Google Scholar] [CrossRef]
- Tan, G.; Zhang, L.; Ning, C.; Liu, X.; Liao, J. Preparation and Characterization of APTES Films on Modification Titanium by SAMs. Thin Solid Films 2011, 519, 4997–5001. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. APTES-modified Mesoporous Silicas as the Carriers for Poorly Water-soluble Drug. Modeling of Diflunisal Adsorption and Release. Appl. Surf. Sci. 2016, 368, 348–359. [Google Scholar] [CrossRef]
- Ten, S.T.; Hashim, U.; Gopinath, S.C.B.; Liu, W.W.; Foo, K.L.; Sam, S.T.; Rahman, S.F.A.; Voon, C.H.; Nordin, A.N. Highly Sensitive Escherichia coli Shear Horizontal Surface Acoustic Wave Biosensor with Silicon Dioxide Nanostructures. Biosens. Bioelectron. 2017, 93, 146–154. [Google Scholar] [CrossRef]
- Khaldi, K.; Sam, S.; Lounas, A.; Yaddaden, C.; Gabouze, N.-E. Comparative Investigation of Two Methods for Acetylcholinesterase Enzyme Immobilization on Modified Porous Silicon. Appl. Surf. Sci. 2017, 421, 148–154. [Google Scholar] [CrossRef]
- Sun, Y.; Yanagisawa, M.; Kunimoto, M.; Nakamura, M.; Homma, T. Depth Profiling of APTES Self-assembled Monolayers Using Surface-enhanced Confocal Raman Microspectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 184, 1–6. [Google Scholar] [CrossRef]
- Leite, A.J.B.; Lima, E.C.; dos Reis, G.S.; Thue, P.S.; Saucier, C.; Rodembusch, F.S.; Dias, S.L.P.; Umpierres, C.S.; Dotto, G.L. Hybrid Adsorbents of Tannin and APTES (3-aminopropyltriethoxysilane) and Their Application for the Highly Efficient Removal of Acid Red 1 Dye from Aqueous Solutions. J. Environ. Chem. Eng. 2017, 5, 4307–4318. [Google Scholar] [CrossRef]
- Coombs, S.G.; Khodjaniyazova, S.; Bright, F.V. Exploiting the 3-Aminopropyltriethoxysilane (APTES) Autocatalytic Nature to Create Bioconjugated Microarrays on Hydrogen-passivated Porous Silicon. Talanta 2018, 177, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Victor dos Santos Junior, C.; Sader, M.S.; Gonçalves, G.C.; Weissmüller, G.; Simão, R.A. Effect of pH on the Adsorption and Interactions of Bovine Serum Albumin with Functionalized Silicon Nitride Surface. Colloids Surf. B Biointerfaces 2018, 167, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Saengdee, P.; Promptmas, C.; Thanapitak, S.; Srisuwan, A.; Pankiew, A.; Thornyanadacha, N.; Chaisriratanakul, W.; Chaowicharat, E.; Jeamsaksiri, W. Optimization of 3-aminopropyltriethoxysilane Functionalization on Silicon Nitride Surface for Biomolecule Immobilization. Talanta 2020, 207, 120305. [Google Scholar] [CrossRef]
- Acres, R.G.; Ellis, A.V.; Alvino, J.; Lenahan, C.E.; Khodakov, D.A.; Metha, G.F.; Andersson, G.G. Molecular Structure of 3-Aminopropyltriethoxysilane Layers Formed on Silanol-Terminated Silicon Surfaces. J. Phys. Chem. C 2012, 116, 6289–6297. [Google Scholar] [CrossRef]
- Chhasatia, R.; Sweetman, M.J.; Harding, F.J.; Waibel, M.; Kay, T.; Thomas, H.; Loudovaris, T.; Voelcker, N.H. Non-invasive, in vitro Analysis of Islet Insulin Production Enabled by an Optical Porous Silicon Biosensor. Biosens. Bioelectron. 2017, 91, 515–522. [Google Scholar] [CrossRef]
- Saengdee, P.; Chaisriratanakul, W.; Bunjongpru, W.; Sripumkhai, W.; Srisuwan, A.; Hruanun, C.; Poyai, A.; Phunpae, P.; Pata, S.; Jeamsaksiri, W.; et al. A Silicon Nitride ISFET Based Immunosensor for Ag85B Detection of Tuberculosis. Analyst 2016, 141, 5767–5775. [Google Scholar] [CrossRef]
- Tsai, T.-C.; Liu, C.-W.; Wu, Y.-C.; Ondevilla, N.A.P.; Osawa, M.; Chang, H.-C. In situ Study of EDC/NHS Immobilization on Gold Surface Based on Attenuated Total Reflection Surface-enhanced Infrared Absorption Spectroscopy (ATR-SEIRAS). Colloids Surf. B Biointerfaces 2019, 175, 300–305. [Google Scholar] [CrossRef]
- Damsongsang, P.; Chaikiawkeaw, D.; Phoolcharoen, W.; Rattanapisit, K.; Kaewpungsup, P.; Pavasant, P.; Hoven, V.P. Surface-immobilized Plant-derived Osteopontin as an Effective Platform to Promote Osteoblast Adhesion and Differentiation. Colloids Surf. B Biointerfaces 2019, 173, 816–824. [Google Scholar] [CrossRef]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and Characterization of Immobilized Antibodies for Improved Immunoassays. Biointerphases 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Park, C.; Kwon, D.; Kim, D.; Meyyappan, M.; Jeon, S.; Lee, J.-S. Silicon Nanowire Biosensors for Detection of Cardiac Troponin I (cTnI) with High Sensitivity. Biosens. Bioelectron. 2016, 77, 695–701. [Google Scholar] [CrossRef]
- Jian-Jun, H. Highly-sensitive Optical Biosensors Based on Silicon-on-insulator Nanowire Waveguide. In Proceedings of the 2016 Progress in Electromagnetic Research Symposium (PIERS), Shanghai, China, 8–11 August 2016; p. 1615. [Google Scholar]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Mahmudin, D.; Estu, T.T.; Daud, P.; Hermida, I.D.P.; Sugandi, G.; Wijayanto, Y.N.; Menon, P.S.; Shaari, S. Sensitivity Improvement of Multipath Optical Ring Resonators Using Silicon-on-insulator Technology. In Proceedings of the 2015 IEEE Regional Symposium on Micro and Nanoelectronics (RSM), Kuala Terengganu, Malaysia, 19–21 August 2015; pp. 1–4. [Google Scholar]
- Ghasemi, F.; Eftekhar, A.A.; Mousavi, S.H.S.; Abbaspour, R.; Moradinejad, H.; Adibi, A. Lab-on-chip Silicon Nitride Microring Sensor at Visiblewavelength Using Glycoprotein Receptors. In Proceedings of the 2014 Conference on Lasers and Electro-Optics (CLEO)–Laser Science to Photonic Applications, San Jose, CA, USA, 8–13 June 2014; pp. 1–2. [Google Scholar]
- Balakrishnan, S.R.; Hashim, U.; Letchumanan, G.R.; Kashif, M.; Ruslinda, A.R.; Liu, W.W.; Veeradasan, P.; Haarindra Prasad, R.; Foo, K.L.; Poopalan, P. Development of Highly Sensitive Polysilicon Nanogap with APTES/GOx Based Lab-on-chip Biosensor to Determine Low Levels of Salivary Glucose. Sens. Actuators A Phys. 2014, 220, 101–111. [Google Scholar] [CrossRef]
- Zhang, B.; Tamez-Vela, J.M.; Solis, S.; Bustamante, G.; Peterson, R.; Rahman, S.; Morales, A.; Tang, L.; Ye, J.Y. Detection of Myoglobin with an Open-Cavity-Based Label-Free Photonic Crystal Biosensor. J. Med. Eng. 2013. [Google Scholar] [CrossRef]
- Lasmi, K.; Derder, H.; Kermad, A.; Sam, S.; Boukhalfa-Abib, H.; Belhousse, S.; Tighilt, F.Z.; Hamdani, K.; Gabouze, N. Tyrosinase Immobilization on Functionalized Porous Silicon Surface for Optical Monitoring of Pyrocatechol. Appl. Surf. Sci. 2018, 446, 3–9. [Google Scholar] [CrossRef]
- Chhasatia, R.; Sweetman, M.J.; Prieto-Simon, B.; Voelcker, N.H. Performance Optimisation of Porous Silicon Rugate Filter Biosensor for the Detection of Insulin. Sens. Actuators B Chem. 2018, 273, 1313–1322. [Google Scholar] [CrossRef]
- Wang, S.; Shan, X.; Patel, U.; Huang, X.; Lu, J.; Li, J.; Tao, N. Label-free Imaging, Detection, and Mass Measurement of Single Viruses by Surface Plasmon Resonance. Proc. Natl. Acad. Sci. USA 2010, 107, 16028–16032. [Google Scholar] [CrossRef] [Green Version]
- Ladd, J.; Taylor, A.D.; Piliarik, M.; Homola, J.; Jiang, S.Y. Label-free Detection of Cancer Biomarker Candidates Using Surface Plasmon Resonance Imaging. Anal. Bioanal. Chem. 2009, 393, 1157–1163. [Google Scholar] [CrossRef]
- Carrara, S.; Cavallini, A.; Maruyama, Y.; Charbon, E.; De Micheli, G. A New Ethylene Glycol-silane Monolayer for Highly-specific DNA Detection on Silicon Chips. Surf. Sci. 2010, 604, L71–L74. [Google Scholar] [CrossRef] [Green Version]
- Staros, J.V.; Wright, R.W.; Swingle, D.M. Enhancement by N-hydroxysulfosuccinimide of Water-soluble Carbodiimide-mediated Coupling Reactions. Anal. Biochem. 1986, 156, 220–222. [Google Scholar] [CrossRef]
- Mendez-Astudillo, M.; Takahisa, H.; Fujiwara, K.; Okayama, H.; Nakajima, H. Multimode Rectangular Optical Microcavity for Biomarker Detection Based on Silicon on Insulator. In Proceedings of the 2016 Conference on Lasers and Electro-Optics (CLEO), San Jose, CA, USA, 5–10 June 2016; pp. 1–2. [Google Scholar]
- Rendevski, V.; Aleksovski, B.; Stojanov, D.; Mihajlovska-Rendevska, A.; Aleksovski, V.; Baneva-Dolnenec, N.; Nikodijevic, D.; Gudeva-Nikovska, D. Validation of the ELISA Method for Quantitative Detection of TNF-alpha in Patients with Intracerebral Hemorrhage. Open Access Maced. J. Med. Sci. 2017, 5, 703–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udomsom, S.N.I.; Mankong, U. AFM Study on Protein Coated Silicon Surface Morphology for Label-free Bio-sensing. In Proceedings of the 2018 International Conference on Analog VLSI Circuits (AVIC), Chiang Mai, Thailand, 31 October–2 November 2018. [Google Scholar]
- Berlind, T.; Tengvall, P.; Hultman, L.; Arwin, H. Protein Adsorption on Thin Films of Carbon and Carbon Nitride Monitored with in situ Ellipsometry. Acta Biomater. 2011, 7, 1369–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.H.; Jin, G. Silicon Surface Modification with a Mixed Silanes Layer to Immobilize Proteins for Biosensor with Imaging Ellipsometry. Colloids Surf. B Biointerfaces 2004, 34, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Nehilla, B.J.; Popat, K.C.; Vu, T.Q.; Chowdhury, S.; Standaert, R.F.; Pepperberg, D.R.; Desai, T.A. Neurotransmitter Analog Tethered to a Silicon Platform for Neuro-BioMEMS Applications. Biotechnol. Bioeng. 2004, 87, 669–674. [Google Scholar] [CrossRef]
- Udomsom, S.; Mankong, U.; Theera-Umpon, N.; Ittipratheep, N.; Umezawa, T.; Matsumoto, A.; Yamamoto, N. Silicon Photonic Resonator for Label-free Bio-sensing Application. In Proceedings of the Third International Conference on Photonic Solutions, Pattaya, Thailand, 8–10 November 2017; p. 10. [Google Scholar]
- Voros, J. The Density and Refractive Index of Adsorbing Protein Layers. Biophys. J. 2004, 87, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Berlind, T.; Poksinski, M.; Tengvall, P.; Arwin, H. Formation and Cross-linking of Fibrinogen Layers Monitored with in situ Spectroscopic Ellipsometry. Colloids Surf. B Biointerfaces 2010, 75, 410–417. [Google Scholar] [CrossRef] [Green Version]
- Zaaijer, H.L.; Vrielink, H.; Koot, M. Early Detection of Hepatitis B Surface Antigen and Detection of HBsAg Mutants: A Comparison of Five Assays. Vox Sang. 2001, 81, 219–221. [Google Scholar] [CrossRef]
- Gonzalez, J.; Araya, J.; Olivares, H.; Sagua, H. ELISA Reaction for the Diagnosis of South American Trypanosomiasis. Limit Absorbances for Reacting and Non-reacting Sera. Bol. Chil. Parasitol. 1986, 41, 21–26. [Google Scholar]
- Hayashi, Y.; Matsuda, R.; Maitani, T.; Imai, K.; Nishimura, W.; Ito, K.; Maeda, M. Precision, Limit of Detection and Range of Quantitation in Competitive ELISA. Anal. Chem. 2004, 76, 1295–1301. [Google Scholar] [CrossRef]
- Mosae Selvakumar, P. Phenol Sensing Studies by 4-Aminoantipyrine Method-A Review. Org. Med. Chem. Int. J. 2018, 5. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Optimized Enzymatic Colorimetric Assay for Determination of Hydrogen Peroxide (H2O2) Scavenging Activity of Plant Extracts. MethodsX 2015, 2, 283–291. [Google Scholar] [CrossRef] [PubMed]
- To, T.D.; Nguyen, A.T.; Phan, K.N.T.; Truong, A.T.T.; Doan, T.C.D.; Dang, C.M. Modification of Silicon Nitride Surfaces with GOPES and APTES for Antibody Immobilization: Computational and Experimental Studies. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045006. [Google Scholar] [CrossRef]
- Sharma, A.; Bhat, S.; Vishnoi, T.; Nayak, V.; Kumar, A. Three-Dimensional Supermacroporous Carrageenan-Gelatin Cryogel Matrix for Tissue Engineering Applications. BioMed Res. Int. 2013, 2013, 478279. [Google Scholar] [CrossRef] [PubMed] [Green Version]















| Silicon Protein Coating Technique | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| APTES-GA | Commonly used method | GA can bind to nonspecific proteins; thickness of the APTES layer is uncontrollable | [30,34,35,36,37,38,39] |
| EDC-NHS | Specific binding between EDC and NHS leads to regular molecule arrangement; PEG is hydrophobic | Requires the use of hazardous substance, thus requiring supported laboratory | [31,40,41,42] |
| PEG | The functional groups used in the crosslink may be selected | Large PEG molecules may cause nonuniform coating layer | [29,43,44,45] |
| Layer of Measurement | Root Mean Square (RMS) Roughness (nm) | |
|---|---|---|
| APTES-GA Technique | APTES-(EDC/NHS) Technique | |
| Silicon surface (uncoated) | 0.5 | 0.79 |
| APTES | 1.48 | 1.22 |
| APTES-GA | 1.78 | N/A |
| Anti-TNF-alpha layer | 6.30 | 1.50 |
| GOx layer | 8.70 | 2.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udomsom, S.; Mankong, U.; Paengnakorn, P.; Theera-Umpon, N. Novel Rapid Protein Coating Technique for Silicon Photonic Biosensor to Improve Surface Morphology and Increase Bioreceptor Density. Coatings 2021, 11, 595. https://doi.org/10.3390/coatings11050595
Udomsom S, Mankong U, Paengnakorn P, Theera-Umpon N. Novel Rapid Protein Coating Technique for Silicon Photonic Biosensor to Improve Surface Morphology and Increase Bioreceptor Density. Coatings. 2021; 11(5):595. https://doi.org/10.3390/coatings11050595
Chicago/Turabian StyleUdomsom, Suruk, Ukrit Mankong, Pathinan Paengnakorn, and Nipon Theera-Umpon. 2021. "Novel Rapid Protein Coating Technique for Silicon Photonic Biosensor to Improve Surface Morphology and Increase Bioreceptor Density" Coatings 11, no. 5: 595. https://doi.org/10.3390/coatings11050595
APA StyleUdomsom, S., Mankong, U., Paengnakorn, P., & Theera-Umpon, N. (2021). Novel Rapid Protein Coating Technique for Silicon Photonic Biosensor to Improve Surface Morphology and Increase Bioreceptor Density. Coatings, 11(5), 595. https://doi.org/10.3390/coatings11050595