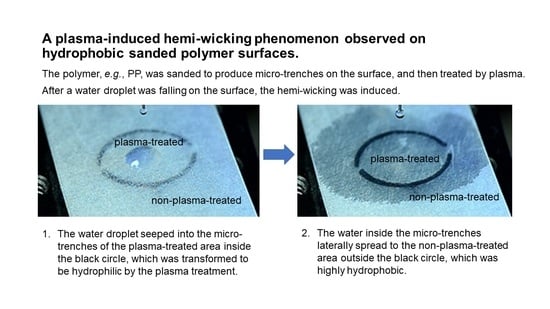
Plasma-Induced Hemi-Wicking on Sanded Polymer Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. APPJ System Configuration and Instrumentation
2.2. Polymer Surface Treatment Test Conditions
2.3. Surface Properties Characterization
2.4. Adhesive Stress Measurement
3. Results
3.1. APPJ Characterization
3.2. Hemi-Wicking
3.3. CA Measurements
3.4. XPS Analysis
3.5. Shear Stress Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhowmik, S.; Ghosh, P.K.; Ray, S. Surface Modification of HDPE and PP by Mechanical Polishing and DC Glow Discharge and Their Adhesive Joining to Steel. J. Appl. Polym. Sci. 2001, 80, 1140–1149. [Google Scholar] [CrossRef]
- Ryntz, R.A. Coating Adhesion to Low Surface Free Energy Substrates. Prog. Org. Coat. 1994, 25, 73–83. [Google Scholar] [CrossRef]
- Kim, S. The Role of Plastic Package Adhesion in IC Performance. In Proceedings of the 1991 Proceedings 41st Electronic Components & Technology Conference, Atlanta, GA, USA, 11–16 May 1991. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Wettability and Surface Free Energy of Corona-Treated Biaxially-Oriented Polypropylene Film. J. Adhes. Sci. Technol. 2001, 15, 1769–1785. [Google Scholar] [CrossRef]
- ASTM D2093-03, Standard Practice for Preparation of Surfaces of Plastics Prior to Adhesive Bonding; ASTM International: West Conshohocken, PA, USA, 2003. [CrossRef]
- Guo, C.; Wang, S.; Liu, H.; Feng, L.; Song, Y.; Jiang, L. Wettability Alteration of Polymer Surfaces Produced by Scraping. J. Adhes. Sci. Technol. 2008, 22, 395–402. [Google Scholar] [CrossRef]
- Cho, D.L.; Shin, K.H.; Lee, W.-J.; Kim, D.-H. Improvement of Paint Adhesion to a Polypropylene Bumper by Plasma Treatment. J. Adhes. Sci. Technol. 2001, 15, 653–664. [Google Scholar] [CrossRef]
- Deynse, A.V.; Cools, P.; Leys, C.; Morent, R.; Geyter, N.D. Surface Modification of Polyethylene in an Argon Atmospheric Pressure Plasma Jet. Surf. Coat. Technol. 2015, 276, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Schulz, U.; Munzert, P.; Kaiser, N. Surface Modification of PMMA by DC Glow Discharge and Microwave Plasma Treatment for the Improvement of Coating Adhesion. Surf. Coat. Tech. 2001, 142–144, 507–511. [Google Scholar] [CrossRef]
- Lai, J.; Sunderland, B.; Xue, J.; Yan, S.; Zhao, W.; Folkard, M.; Michael, B.D.; Wang, Y. Study on Hydrophilicity of Polymer Surfaces Improved by Plasma Treatment. Appl. Surf. Sci. 2006, 252, 3375–3379. [Google Scholar] [CrossRef]
- Poncin-Epaillard, F.; Chevet, B.; Brosse, J.-C. Modification of Isotactic Poly (Propylene) with a Nitrogen Plasma; Differences in Comparison to the Treatment with a Carbon Dioxide Plasma. Makromol. Chem. 1991, 192, 1589–1599. [Google Scholar] [CrossRef]
- Hall, J.R.; Westerdahl, C.A.L.; Devine, A.T.; Bodnar, M.J. Activated Gas Plasma Surface Treatment of Polymers for Adhesive Bonding. J. Appl. Polym. Sci. 1969, 13, 2085–2096. [Google Scholar] [CrossRef]
- Gonzalez, E.I.; Barankin, M.D.; Guschl, P.C.; Hicks, R.F. Surface Activation of Poly (Methyl Methacrylate) via Remote Atmospheric Pressure Plasma. Plasma Process Polym. 2010, 7, 482–493. [Google Scholar] [CrossRef]
- Yasuda, H.K.; Sharma, A.K.; Hale, E.B.; James, W.J. Atomic Interfacial Mixing to Create Water Insensitive Adhesion. J. Adhes. 1982, 13, 269–283. [Google Scholar] [CrossRef]
- Anand, M.; Cohen, R.E.; Baddour, R.F. Surface Modification of Low Density Polyethylene in a Fluorine Gas Plasma. Polymer 1981, 22, 361–371. [Google Scholar] [CrossRef]
- Chiang, M.-H.; Liao, K.-C.; Lin, I.-M.; Lu, C.-C.; Huang, H.-Y.; Kuo, C.-L.; Wu, J.-S. Modification of Hydrophilic Property of Polypropylene Films by a Parallel-Plate Nitrogen-Based Dielectric Barrier Discharge Jet. IEEE Trans Plasma Sci. 2010, 38, 1489–1498. [Google Scholar] [CrossRef]
- Kim, J.; Moon, M.-W.; Kim, H.-Y. Dynamics of Hemiwicking. J. Fluid Mech. 2016, 800, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Bico, J.; Thiele, U.; Quéré, D. Wetting of Textured Surfaces. Colloids Surf. A Phys. Eng. Asp. 2002, 206, 41–46. [Google Scholar] [CrossRef]
- Quéré, D. Rough Ideas on Wetting. Physica A 2002, 313, 32–46. [Google Scholar] [CrossRef]
- Spori, D.M.; Drobek, T.; Zürcher, S.; Ochsner, M.; Sprecher, C.; Mühlebach, A.; Spencer, N.D. Beyond the Lotus Effect: Roughness Influences on Wetting over a Wide Surface-Energy Range. Langmuir 2008, 24, 5411–5417. [Google Scholar] [CrossRef]
- Chen, H.; Zang, H.; Li, X.; Zhao, Y. Toward a Better Understanding of Hemiwicking: A Simple Model to Comprehensive Prediction. Langmuir 2019, 35, 2854–2864. [Google Scholar] [CrossRef]
- Kim, B.S.; Choi, G.; Shim, D.I.; Kim, K.M.; Cho, H.H. Surface Roughening for Hemi-Wicking and Its Impact on Convective Boiling Heat Transfer. Int. J. Heat Mass. Transf. 2016, 102, 1100–1107. [Google Scholar] [CrossRef]
- Ghosh, A.; Beaini, S.; Zhang, B.J.; Ganguly, R.; Megaridis, C.M. Enhancing Dropwise Condensation through Bioinspired Wettability Patterning. Langmuir 2014, 30, 13103–13115. [Google Scholar] [CrossRef]
- Wu, J.-S.; Liu, C.-T.; Hsiao, C.-P.; Wu, M.-C. Atmospheric-Pressure Plasma Jet Generating Device. U.S. Patent 10,121,638, 6 November 2018. [Google Scholar]
- ASTM D3163-01, Standard Test Method for Determining Strength of Adhesively Bonded Rigid Plastic Lap-Shear Joints in Shear by Tension Loading; ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- Morent, R.; Geyter, N.D.; Leys, C.; Gengembre, L.; Payen, E. Study of the Ageing Behaviour of Polymer Films Treated with a Dielectric Barrier Discharge in Air, Helium and Argon at Medium Pressure. Surf. Coat. Tech. 2007, 201, 7847–7854. [Google Scholar] [CrossRef]
- Hegemann, D.; Brunner, H.; Oehr, C. Plasma Treatment of Polymers for Surface and Adhesion Improvement. Nucl. Instrum. Methods Phys. Res. B 2003, 208, 281–286. [Google Scholar] [CrossRef]
- Occhiello, E.; Morra, M.; Cinquina, P.; Garbassi, F. Hydrophobic Recovery of Oxygen-Plasma-Treated Polystyrene. Polymer 1992, 33, 3007–3015. [Google Scholar] [CrossRef]
- Takke, V.; Behary, N.; Perwuelz, A.; Campagne, C. Studies on the Atmospheric Air-Plasma Treatment of PET (Polyethylene Terephtalate) Woven Fabrics: Effect of Process Parameters and of Aging. J. Appl. Polym. Sci. 2009, 114, 348–357. [Google Scholar] [CrossRef]
- Gotoh, K.; Shohbuke, E.; Kobayashi, Y.; Yamada, H. Wettability Control of PET Surface by Plasma-Induced Polymer Film Deposition and Plasma/UV Oxidation in Ambient Air. Colloids Surf. A Phys. Eng. Asp. 2018, 556, 1–10. [Google Scholar] [CrossRef]
- Wagner, H.-E.; Brandenburg, R.; Kozlov, K.V.; Sonnenfeld, A.; Michel, P.; Behnke, J.F. The Barrier Discharge: Basic Properties and Applications to Surface Treatment. Vacuum 2003, 71, 417–436. [Google Scholar] [CrossRef] [Green Version]
- Morent, R.; Geyter, N.D.; Gengembre, L.; Leys, C.; Payen, E.; Vlierberghe, S.V.; Schacht, E. Surface Treatment of a Polypropylene Film with a Nitrogen DBD at Medium Pressure. Eur. Phys. J. Appl. Phys. 2008, 43, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-C.; Liu, C.-T.; Chiang, C.-Y.; Lin, Y.-J.; Lin, Y.-H.; Chang, Y.-W.; Wu, J.-S. Inactivation Effect of Colletotrichum Gloeosporioides by Long-Lived Chemical Species Using Atmospheric-Pressure Corona Plasma-Activated Water. IEEE Trans Plasma Sci. 2019, 47, 1100–1104. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Cheng, K.-Y.; Cheng, Y.-P.; Tschang, C.-Y.T.; Chiu, H.-Y.; Yeh, N.-L.; Liao, K.-C.; Gu, B.-R.; Wu, J.-S. Acute Rat Cutaneous Wound Healing for Small and Large Wounds Using Ar/O2 Atmospheric-Pressure Plasma Jet Treatment. Plasma Med. 2017, 7, 227–243. [Google Scholar] [CrossRef]
- Cheng, K.-Y.; Lin, Z.-H.; Cheng, Y.-P.; Chiu, H.-Y.; Yeh, N.-L.; Wu, T.-K.; Wu, J.-S. Wound Healing in Streptozotocin-Induced Diabetic Rats Using Atmospheric-Pressure Argon Plasma Jet. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Asfardjani, K.; Segui, Y.; Aurelle, Y.; Abidine, N. Effect of Plasma Treatments on Wettability of Polysulfone and Polyetherimide. J. Appl. Polym. Sci. 1991, 43, 271–281. [Google Scholar] [CrossRef]
- Kamal, S.A.A.; Ritikos, R.; Rahman, S.A. Wetting Behaviour of Carbon Nitride Nanostructures Grown by Plasma Enhanced Chemical Vapour Deposition Technique. Appl. Surf. Sci. 2015, 328, 146–153. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Cell Adhesion to Plasma-Treated Polymer Surfaces. Polymer 1993, 34, 2208–2212. [Google Scholar] [CrossRef]
- Williams, T.S.; Yu, H.; Yeh, P.-C.; Yang, J.-M.; Hicks, R.F. Atmospheric Pressure Plasma Effects on the Adhesive Bonding Properties of Stainless Steel and Epoxy Composites. J. Compos Mater 2012, 48, 219–233. [Google Scholar] [CrossRef]
- Banerjee, S.; Tyagi, A.K. Functional Materials; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Han, S.; Lee, Y.; Kim, H.; Kim, G.; Lee, J.; Yoon, J.-H.; Kim, G. Polymer Surface Modification by Plasma Source Ion Implantation. Surf. Coat. Technol. 1997, 93, 261–264. [Google Scholar] [CrossRef]
- Liston, E.M.; Martinu, L.; Wertheimer, M.R. Plasma Surface Modification of Polymers for Improved Adhesion: A Critical Review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Riccardi, C.; Barni, R.; Selli, E.; Mazzone, G.; Massafra, M.R.; Marcandalli, B.; Poletti, G. Surface Modification of Poly (Ethylene Terephthalate) Fibers Induced by Radio Frequency Air Plasma Treatment. Appl. Surf. Sci. 2003, 211, 386–397. [Google Scholar] [CrossRef]
- Sarani, A.; Nikiforov, A.Y.; Geyter, N.D.; Morent, R.; Leys, C. Surface Modification of Polypropylene with an Atmospheric Pressure Plasma Jet Sustained in Argon and an Argon/Water Vapour Mixture. Appl. Surf. Sci. 2011, 257, 8737–8741. [Google Scholar] [CrossRef]
- Fang, Z.; Yang, J.; Liu, Y.; Shao, T.; Zhang, C. Surface Treatment of Polyethylene Terephthalate to Improving Hydrophilicity Using Atmospheric Pressure Plasma Jet. IEEE Trans Plasma Sci. 2013, 41, 1627–1634. [Google Scholar] [CrossRef]
- Iqbal, M.; Dinh, D.K.; Abbas, Q.; Imran, M.; Sattar, H.; Ahmad, A.U. Controlled Surface Wettability by Plasma Polymer Surface Modification. Surfaces 2019, 2, 349–371. [Google Scholar] [CrossRef] [Green Version]
- Cui, N.-Y.; Brown, N.M.D. Modification of the Surface Properties of a Polypropylene (PP) Film Using an Air Dielectric Barrier Discharge Plasma. Appl. Surf. Sci. 2002, 189, 31–38. [Google Scholar] [CrossRef]
- Strobel, M.; Walzak, M.J.; Hill, J.M.; Lin, A.; Karbashewski, E.; Lyons, C.S. A Comparison of Gas-Phase Methods of Modifying Polymer Surfaces. J. Adhes. Sci. Technol. 1995, 9, 365–383. [Google Scholar] [CrossRef]
- Strobel, M.; Jones, V.; Lyons, C.S.; Ulsh, M.; Kushner, M.J.; Dorai, R.; Branch, M.C. A Comparison of Corona-Treated and Flame-Treated Polypropylene Films. Plasma Polym. 2003, 8, 61–95. [Google Scholar] [CrossRef]














| No. | Polymer | Treatment | C1s (%) | N1s (%) | O1s (%) | O/C (%) | C–C (%) | C–O (%) | C=O (%) | O–C=O (%) | WCA (°) | Shear Stress (Newtons) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PP | – | 85.61 | 0 | 14.39 | 16.81 | 94.21 | 4.53 | 1.24 | 0 | 100.17 | 68.2 ± 6.3 |
| 2 | Plasma | 80.16 | 0 | 19.84 | 24.75 | 93.80 | 5.24 | 0.95 | 0 | 30.8 | 133.0 ± 11.2 | |
| 3 | Sanding | 89.25 | 0 | 10.75 | 12.04 | 92.75 | 5.64 | 1.61 | 0 | 140.88 | 109.1 ± 9.4 | |
| 4 | Both | 68.33 | 1.73 | 29.95 | 43.83 | 78.21 | 9.78 | 6.26 | 5.74 | 0 | 153.7 ± 13.8 | |
| 5 | PET | – | 72.15 | 0 | 27.85 | 38.60 | 74.31 | 14.39 | 0 | 11.30 | 74.42 | 169.8 ± 15.2 |
| 6 | Plasma | 63.17 | 0.93 | 35.90 | 56.83 | 59.03 | 22.02 | 0 | 18.95 | 8.78 | 339.8 ± 31.1 | |
| 7 | Sanding | 73.37 | 0 | 26.63 | 36.29 | 70.62 | 16.73 | 0 | 12.65 | 78.77 | 259.9 ± 22.4 | |
| 8 | Both | 59.57 | 1.24 | 39.19 | 65.79 | 55.53 | 23.86 | 0 | 20.60 | 0 | 331.8 ± 29.9 | |
| 9 | PE | – | 88.08 | 0 | 11.92 | 13.53 | 96.32 | 3.68 | 0 | 0 | 77.53 | 97.1 ± 8.7 |
| 10 | Plasma | 71.82 | 2.18 | 26.00 | 36.20 | 81.61 | 8.08 | 4.80 | 5.51 | 24.86 | 263.5 ± 23.5 | |
| 11 | Sanding | 81.21 | 0 | 18.79 | 23.13 | 87.53 | 8.92 | 2.35 | 1.21 | 103.6 | 192.1 ± 16.8 | |
| 12 | Both | 69.01 | 3.35 | 27.64 | 40.05 | 77.31 | 9.91 | 6.14 | 6.63 | 0 | 384.4 ± 35.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, P.-H.; Wu, C.-J.; Hsiao, C.-P.; Liu, C.-T.; Wu, M.-C.; Wu, J.-S. Plasma-Induced Hemi-Wicking on Sanded Polymer Surfaces. Coatings 2021, 11, 871. https://doi.org/10.3390/coatings11080871
Chiu P-H, Wu C-J, Hsiao C-P, Liu C-T, Wu M-C, Wu J-S. Plasma-Induced Hemi-Wicking on Sanded Polymer Surfaces. Coatings. 2021; 11(8):871. https://doi.org/10.3390/coatings11080871
Chicago/Turabian StyleChiu, Po-Hsien, Ching-Jung Wu, Chun-Ping Hsiao, Chih-Tung Liu, Mu-Chien Wu, and Jong-Shinn Wu. 2021. "Plasma-Induced Hemi-Wicking on Sanded Polymer Surfaces" Coatings 11, no. 8: 871. https://doi.org/10.3390/coatings11080871
APA StyleChiu, P. -H., Wu, C. -J., Hsiao, C. -P., Liu, C. -T., Wu, M. -C., & Wu, J. -S. (2021). Plasma-Induced Hemi-Wicking on Sanded Polymer Surfaces. Coatings, 11(8), 871. https://doi.org/10.3390/coatings11080871