Core/Shell Glycine-Polyvinyl Alcohol/Polycaprolactone Nanofibrous Membrane Intended for Guided Bone Regeneration: Development and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
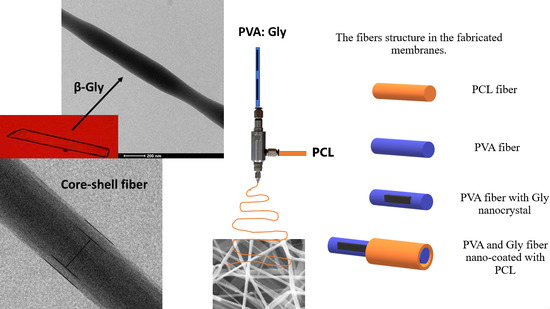
2.2.1. Preparation and Electrospinning of Membranes
2.2.2. Characterization
- SEM analysis.
- TEM analysis.
- Tensile test.
- XPS analysis.
- XRD analysis.
- TGA.
- Wettability.
- In vitro degradation analysis.
- Absorption and shrinkage tests.
- Statistical analysis.
3. Results
3.1. Morphological and Structural Characterization
3.2. Mechanical Characterization
3.3. Chemical Characterization
3.3.1. XPS Characterization
3.3.2. XRD Characterization
3.4. Thermal Characterization
3.5. Physical Characterization
3.5.1. Wettability
3.5.2. In Vitro Degradation
3.5.3. Absorption and Shrinkage Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Gozutok, M.; Basar, A.O.; Sasmazel, H.T. Development of Antibacterial Composite Electrospun Chitosan-Coated Polypropylene Materials. J. Nanosci. Nanotechnol. 2018, 18, 2881–2891. [Google Scholar] [CrossRef]
- Laney, W.R. Glossary of Oral and Maxillofacial Implants. Int. J. Oral Maxillofac. Implant. 2017, 32. [Google Scholar] [CrossRef]
- Zupancic, S.; Kocbek, P.; Baumgartner, S.; Kristl, J. Contribution of Nanotechnology to Improved Treatment of Periodontal Disease. Curr. Pharm. Des. 2015, 21, 3257–3271. [Google Scholar] [CrossRef]
- Basar, A.O.; Sadhu, V.; Turkoglu Sasmazel, H. Preparation of Electrospun PCL-Based Scaffolds by Mono/Multi-Functionalized GO. Biomed. Mater. 2019, 14, 045012. [Google Scholar] [CrossRef] [PubMed]
- Surucu, S.; Sasmazel, H.T. DBD Atmospheric Plasma-Modified, Electrospun, Layer-by-Layer Polymeric Scaffolds for L929 Fibroblast Cell Cultivation. J. Biomater. Sci. Polym. Ed. 2016, 27, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Sasmazel, H.T. Novel Hybrid Scaffolds for the Cultivation of Osteoblast Cells. Int. J. Biol. Macromol. 2011, 49, 838–846. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes—Different Cell Effects. Cytotechnology 2015, 68, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surucu, S.; Turkoglu Sasmazel, H. Development of Core-Shell Coaxially Electrospun Composite PCL/Chitosan Scaffolds. Int. J. Biol. Macromol. 2016, 92, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, O.; Sasmazel, H.T. Antibacterial Performance of PCL-Chitosan Core-Shell Scaffolds. J. Nanosci. Nanotechnol. 2018, 18, 2415–2421. [Google Scholar] [CrossRef]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and Options Using Resorbable versus Nonresorbable Membranes for Successful Guided Bone Regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef]
- Toledano, M.; Gutierrez-Pérez, J.L.; Gutierrez-Corrales, A.; Serrera-Figallo, M.A.; Toledano-Osorio, M.; Rosales-Leal, J.I.; Aguilar, M.; Osorio, R.; Torres-Lagares, D. Novel Non-Resorbable Polymeric-Nanostructured Scaffolds for Guided Bone Regeneration. Clin. Oral. Investig. 2020, 24, 2037–2049. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric Membranes for Guided Bone Regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymer 2016, 8, 115. [Google Scholar] [CrossRef]
- Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. L-Glycine: A Novel Antiinflammatory, Immunomodulatory, and Cytoprotective Agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine Metabolism in Animals and Humans: Implications for Nutrition and Health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Guerin, S.; Stapleton, A.; Chovan, D.; Mouras, R.; Gleeson, M.; Mckeown, C.; Noor, M.; Silien, C.; Rhen, F.; Kholkin, A.; et al. Control of Piezoelectricity in Amino Acids by Supramolecular Packing. Nat. Mater. 2018, 17, 180–186. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Manjakkal, L.; Shakthivel, D.; Dahiya, R. Glycine-Chitosan-Based Flexible Biodegradable Piezoelectric Pressure Sensor. ACS Appl. Mater. Interfaces 2020, 12, 9008–9016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, E.S.; Dahiya, R. Biodegradable Amino Acid-Based Pressure Sensor. In Proceedings of the 2020 IEEE Sensors, online, 25–28 October 2020; pp. 1–4. [Google Scholar]
- Hosseini, E.S.; Manjakkal, L.; Shakthivel, D.; Dahiya, R. Glycine-Based Flexible Biocompatible Piezoelectric Pressure Sensor for Healthcare Applications. In Proceedings of the 2020 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 16–19 August 2020; pp. 1–4. [Google Scholar]
- Mohamed, R.M.; Yusoh, K. A Review on the Recent Research of Polycaprolactone (PCL). Adv. Mater. Res. 2016, 1134, 249–255. [Google Scholar] [CrossRef]
- Gozutok, M.; Sadhu, V.; Sasmazel, H.T. Development of Poly(Vinyl Alcohol) (PVA)/Reduced Graphene Oxide (RGO) Electrospun Mats. J. Nanosci. Nanotechnol. 2019, 19, 4292–4298. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, C.K.; DeForest, C.A. Chapter 19—Polymer Design and Development. In Biology and Engineering of Stem Cell Niches; Vishwakarma, A., Karp, J.M., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 295–314. ISBN 978-0-12-802734-9. [Google Scholar]
- Alhosseini, S.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and Characterization of Electrospun Polyvinyl Alcohol Nanofibrous Scaffolds Modified by Blending with Chitosan for Neural Tissue Engineering. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Mc, P.; Wc, C.; Jm, G.; Ga, C.; Sl, B. Increasing the Pore Sizes of Bone-Mimetic Electrospun Scaffolds Comprised of Polycaprolactone, Collagen I and Hydroxyapatite to Enhance Cell Infiltration. Biomaterials 2011, 33, 524–534. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lou, T.; Zhao, W.; Song, G.; Li, C.; Cui, G. The Effect of Fiber Size and Pore Size on Cell Proliferation and Infiltration in PLLA Scaffolds on Bone Tissue Engineering. J. Biomater. Appl. 2016, 30, 1545–1551. [Google Scholar] [CrossRef]
- de Santana, R.B.; de Mattos, C.M.L.; Francischone, C.E.; Van Dyke, T. Superficial Topography and Porosity of an Absorbable Barrier Membrane Impacts Soft Tissue Response in Guided Bone Regeneration. J. Periodontol. 2010, 81, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Steckl, A. Coaxial Electrospinning Formation of Complex Polymer Fibers and Their Applications. ChemPlusChem 2019, 84, 1451. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, E.S.; Davey, R.J.; Cross, W.I.; Gillon, A.L.; Towler, C.S. Crystallization in Polymorphic Systems: The Solution-Mediated Transformation of β to α Glycine. Cryst. Growth Des. 2003, 3, 53–60. [Google Scholar] [CrossRef]
- Bai, C.; Wang, C.; Tengfei, Z.; Hu, Q. Growth of β-Glycine Crystals Promoted by Standing Surface Acoustic Waves (SSAWs). CrystEngComm 2018, 20, 1245–1251. [Google Scholar] [CrossRef]
- Raz, P.; Brosh, T.; Ronen, G.; Tal, H. Tensile Properties of Three Selected Collagen Membranes. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Cho, S.J.; Jung, S.M.; Kang, M.; Shin, H.S.; Youk, J.H. Preparation of Hydrophilic PCL Nanofiber Scaffolds via Electrospinning of PCL/PVP-b-PCL Block Copolymers for Enhanced Cell Biocompatibility. Polymer 2015, 69, 95–102. [Google Scholar] [CrossRef]
- Kim, D.; Han, S.A.; Kim, J.H.; Lee, J.-H.; Kim, S.-W.; Lee, S.-W. Biomolecular Piezoelectric Materials: From Amino Acids to Living Tissues. Adv. Mater. 2020, 32, 1906989. [Google Scholar] [CrossRef]
- Reddy, M.O.; Chandra Babu, B. Structural, Optical, Electrical, and Magnetic Properties of PVA:Gd3+ and PVA:Ho3+ Polymer Films. Indian J. Mater. Sci. 2015, 2015, e927364. [Google Scholar] [CrossRef] [Green Version]
- Bang, H.; Watanabe, K.; Nakashima, R.; Kai, W.; Song, K.-H.; Lee, J.S.; Gopiraman, M.; Kim, I.-S. A Highly Hydrophilic Water-Insoluble Nanofiber Composite as an Efficient and Easily-Handleable Adsorbent for the Rapid Adsorption of Cesium from Radioactive Wastewater. RSC Adv. 2014, 4, 59571–59578. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Kadota, K.; Shirakawa, Y.; Tozuka, Y.; Shimosaka, A.; Hidaka, J. Morphology Control of Amino Acid Particles in Interfacial Crystallization Using Inkjet Nozzle. Adv. Powder Technol. 2014, 25, 847–852. [Google Scholar] [CrossRef]
- Ouyang, L.; Zheng, T.; Shen, L. Direct Observation of α- to β-Glycine Transformation during the Ionic Liquid-Mediated Crystallization Process. CrystEngComm 2018, 20, 2705–2712. [Google Scholar] [CrossRef]
- Isakov, D.; Gomes, E.d.M.; Bdikin, I.; Almeida, B.; Belsley, M.; Costa, M.; Rodrigues, V.; Heredia, A. Production of Polar β-Glycine Nanofibers with Enhanced Nonlinear Optical and Piezoelectric Properties. Cryst. Growth Des. 2011, 11, 4288–4291. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Turng, L.-S.; Li, Q. Crystalline Morphology of Electrospun Poly(ε-Caprolactone) (PCL) Nanofibers. Ind. Eng. Chem. Res. 2013, 52, 4939–4949. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Gambhir, S.; Officer, D.L.; Wallace, G.G. Covalently Linked Biocompatible Graphene/Polycaprolactone Composites for Tissue Engineering. Carbon 2013, 52, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Weiss, I.M.; Muth, C.; Drumm, R.; Kirchner, H.O.K. Thermal Decomposition of the Amino Acids Glycine, Cysteine, Aspartic Acid, Asparagine, Glutamic Acid, Glutamine, Arginine and Histidine. BMC Biophys 2018, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Turkoglu Sasmazel, H.; Alazzawi, M.; Kadim Abid Alsahib, N. Atmospheric Pressure Plasma Surface Treatment of Polymers and Influence on Cell Cultivation. Molecules 2021, 26, 1665. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of Differently Cross-Linked Collagen Membranes: An Experimental Study in the Rat. Clin. Oral Implant. Res. 2005, 16, 369–378. [Google Scholar] [CrossRef] [PubMed]









| Membrane | Applied Voltage (kV) | Needle-to-Collector Distance (cm) | Feeding Rate (µL/min) |
|---|---|---|---|
| PCL | 7 | 10 | 10 |
| PVA | 17 | 15 | 10 |
| PVA:Gly | 11.5 | 15 | 10 |
| Core-shell | 20 | 22 | Core: 27 Shell: 20 |
| Membrane | Average Fiber Diameter (µm) | Average Pore Size (µm) |
|---|---|---|
| PCL | 0.274 ± 0.055 | 2.573 ± 0.016 |
| PVA | 0.447 ± 0.073 | 4.157 ± 0.056 |
| PVA:Gly | 0.402 ± 0.078 | 4.217 ± 0.103 |
| Core-shell | 0.412 ± 0.082 | 6.803 ± 0.035 |
| Membrane | Modulus of Elasticity (MPa) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PCL | 2.86 ± 0.07 | 4.23 ± 0.01 | 120 ± 3 |
| PVA | 49.7 ± 0.556 | 1.19 ± 0.1 | 11.3 ± 2.02 |
| PVA:Gly | 241.3 ± 1.52 | 5.69 ± 0.02 | 19 ± 0.98 |
| Core-shell | 135.3 ± 3.05 | 4.57 ± 0.04 | 39.43 ± 0.58 |
| Membranes | Average C.A | C.A Image |
|---|---|---|
| PCL | 100 ± 8.1° |  |
| PVA | ~0 | − |
| PVA:Gly | ~0 | − |
| Core-shell | 47.4 ± 2.2° |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alazzawi, M.; Kadim Abid Alsahib, N.; Turkoglu Sasmazel, H. Core/Shell Glycine-Polyvinyl Alcohol/Polycaprolactone Nanofibrous Membrane Intended for Guided Bone Regeneration: Development and Characterization. Coatings 2021, 11, 1130. https://doi.org/10.3390/coatings11091130
Alazzawi M, Kadim Abid Alsahib N, Turkoglu Sasmazel H. Core/Shell Glycine-Polyvinyl Alcohol/Polycaprolactone Nanofibrous Membrane Intended for Guided Bone Regeneration: Development and Characterization. Coatings. 2021; 11(9):1130. https://doi.org/10.3390/coatings11091130
Chicago/Turabian StyleAlazzawi, Marwa, Nabeel Kadim Abid Alsahib, and Hilal Turkoglu Sasmazel. 2021. "Core/Shell Glycine-Polyvinyl Alcohol/Polycaprolactone Nanofibrous Membrane Intended for Guided Bone Regeneration: Development and Characterization" Coatings 11, no. 9: 1130. https://doi.org/10.3390/coatings11091130
APA StyleAlazzawi, M., Kadim Abid Alsahib, N., & Turkoglu Sasmazel, H. (2021). Core/Shell Glycine-Polyvinyl Alcohol/Polycaprolactone Nanofibrous Membrane Intended for Guided Bone Regeneration: Development and Characterization. Coatings, 11(9), 1130. https://doi.org/10.3390/coatings11091130