Stability of Supehydrophobic Layers Formed by Organic Acids on the Surface of Aluminum Alloy 6063
Abstract
:1. Introduction
2. Experimental
2.1. Materials
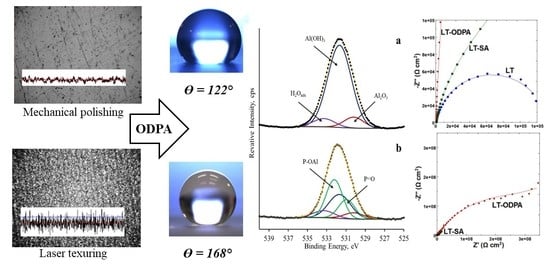
2.2. The Preparation of SHP Surface
- Short-term etching (10 s) in a 10% NaOH solution (analytical grade, RusKhim, Russia) at t = 65 °C, followed by washing the samples with distilled water and drying in air at t = 65 °C.
- Processing with an air-cooled fiber-optic laser marker XM-30 (PPK-Laser, Kazan, Russia) with the following parameters of laser processing (LP): λ—wavelength 1.064 μm, ʋ—radiation frequency (20 kHz), d—laser beam diameter (0.01 mm), l—distance between linear trajectories (0.01 mm), varying ν—the speed of movement of the laser beam (300 ÷ 700 mm/s) and W—laser power (6 ÷ 12 W). LP was performed with a single laser pass, i.e., with obtaining a linear "bed" structure. Furthermore, to remove the metal dust formed during the LP process, the samples were washed with ethanol and dried in air at t = 65 °C.
2.3. Estimation of Surface Roughness Parameters for Alloy 6063 Specimens
2.3.1. Optical Microscopy
2.3.2. Profilometry
2.4. Characterization of SHP Surface Alloy 6063
2.4.1. Water Contact Angle Measurements and Their Temporary Evolution
2.4.2. XPS Studies
2.5. Study of the Protective Properties of SHP Films Formed on Alloy 6063
2.5.1. Polarization and EIS Measurements
2.5.2. Corrosion Test in a Salt Spray Chamber
3. Results and Discussion
3.1. Surface Morphology of Alloy 6063
3.2. SHP State of the Coatings and Its Evolution of during Long-Term Contact with Water Solution
3.3. Polarization Measurements
3.4. EIS
3.5. XPS
3.6. Corrosion Tests and a Degradation of SHP Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control; an Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008; pp. 383–386. [Google Scholar]
- Quebbou, Z.; Chafi, M.; Omari, L.E.H. Corrosion resistance of 5005 aluminum alloy by anodizing treatment in a mixture of phosphoric and boric acids. Mater. Today Proc. 2021, 37, 3854–3859. [Google Scholar] [CrossRef]
- Johansen, H.D.; Brett, C.M.A.; Motheo, A.J. Corrosion protection of aluminium alloy by cerium conversion and conducting polymer duplex coatings. Corros. Sci. 2012, 63, 342–350. [Google Scholar] [CrossRef]
- Babaei, K.; Fattah-alhosseini, A.; Molaei, M. The effects of carbon-based additives on corrosion and wear properties of plasma electrolytic oxidation (PEO) coatings applied on aluminum and its alloys: A review. Surf. Interfaces 2020, 21, 100677. [Google Scholar] [CrossRef]
- Rakoch, A.G.; Gladkova, A.A.; Linn, Z.; Strekalina, D.M. The evidence of cathodic micro-discharges during plasma electrolytic oxidation of light metallic alloys and micro-discharge intensity depending on pH of the electrolyte. Surf. Coat. Technol. 2015, 269, 138–144. [Google Scholar] [CrossRef]
- Thai, T.T.; Druart, M.-E.; Paint, Y.; Trinh, A.T.; Olivier, M.-G. Influence of the sol-gel mesoporosity on the corrosion protection given by an epoxy primer applied on aluminum alloy 2024–T3. Prog. Org. Coat. 2018, 121, 53–63. [Google Scholar] [CrossRef]
- Bouali, A.C.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Kendig, M.W.; Buchheit, R.G. Corrosion inhibition of aluminum and aluminum alloys by soluble chromates, chromate coatings, and chromate-free coatings. Corrosion 2003, 59, 379–400. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Normand, B.; Pagetti, J. EIS characterisation of anodic films formed on 2024 aluminium alloy, in sulphuric acid containing molybdate or permanganate species. Corros. Sci. 2005, 47, 937–951. [Google Scholar] [CrossRef]
- Oleinik, S.V.; Malygina, E.M.; Zimina, Y.M. The effects of temperature on the chemical oxidation of AMg-3 alloy in molybdate-containing solutions. Prot. Met. 2007, 43, 674–678. [Google Scholar] [CrossRef]
- Xhanari, K.; Finsgar, M. Organic corrosion inhibitors for aluminum and its alloys in chloride and alkaline solutions: A review. Arab. J. Chem. 2019, 12, 4646–4663. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I. Triazoles as a class of multifunctional corrosion inhibitors. Review. Part III. 1,2,3-Benzotriazole and its derivatives. Aluminum alloys. Int. J. Corros. Scale Inhib. 2020, 9, 1142–1168. [Google Scholar] [CrossRef]
- Telegdi, J.; Luciano, G.; Mahanty, S.; Abohalkuma, T. Inhibition of aluminum alloy corrosion in electrolytes by self-assembled fluorophosphonic acid molecular layer. Mater. Corros. 2016, 67, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Boisier, G.; Portail, N.; Pebere, N. Corrosion inhibition of 2024 aluminium alloy by sodium decanoate. Electrochim. Acta. 2010, 55, 6182–6189. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef] [Green Version]
- Yao, W.; Wu, L.; Huang, G.; Jiang, B.; Atrens, A.; Pan, F. Superhydrophobic coatings for corrosion protection of magnesium alloys. J. Mater. Sci. Technol. 2020, 52, 100–118. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and Engineering of Superhydrophobic Surfaces: Review of Corrosion Resistance, Chemical and Mechanical Stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Sethi, S.K.; Manik, G. Recent progress in super hydrophobic/hydrophilic self-cleaning surfaces for various industrial applications: A review. Polym.-Plast. Technol. Eng. 2018, 57, 1932–1952. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Aleshkin, A.V.; Emelyanenko, K.A.; Zulkarneev, E.; Kiseleva, I.; Vasiliev, A.; Emelyanenko, A. Effective antibacterial nanotextured surfaces based on extreme wettability and bacteriophage seeding. Appl. Nano Mater. 2018, 1, 1348–1359. [Google Scholar] [CrossRef]
- Li, W.; Zhan, Y.; Yu, S. Applications of superhydrophobic coatings in anti-icing: Theory, mechanisms, impact factors, challenges and perspectives. Prog. Org. Coat. 2021, 152, 106117. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, P.; Zhang, D. Super-hydrophobic film fabricated on aluminium surface as a barrier to atmospheric corrosion in a marine environment. Corr. Sci. 2015, 91, 287–296. [Google Scholar] [CrossRef]
- Zhang, B.; Xua, W.; Zhua, Q.; Sun, Y.; Lia, Y. Mechanically robust superhydrophobic porous anodized AA5083 for marine corrosion protection. Corr. Sci. 2019, 158, 108083. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Guo, Z.; Shi, T.; Yu, J.; Tang, M.; Huang, X. Fabrication of superhydrophobic surface with improved corrosion inhibition on 6061 aluminum alloy substrate. Appl. Surf. Sci. 2015, 342, 76–83. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Shi, T.; Zhang, C.; Zhang, L.C. Facile preparation of superhydrophobic structures on Al alloys surfaces with superior corrosion resistance. Mater. Corros. 2019, 70, 558–565. [Google Scholar] [CrossRef]
- Xie, D.; Li, W. A novel simple approach to preparation of superhydrophobic surfaces of aluminum alloys. Appl. Surf. Sci. 2011, 258, 1004–1007. [Google Scholar] [CrossRef]
- Saleema, N.; Sarkar, D.K.; Gallant, D.; Paynter, R.W.; Chen, X.-G. Chemical nature of superhydrophobic aluminum alloy surfaces produced via a one-step process using fluoroalkyl-silane in a base medium. ACS Appl. Mater. Interfaces 2011, 3, 4775–4781. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhu, K.; Wu, B.; Lou, J.; Zhang, Z.; Chai, G. Influence of structured sidewalls on the wetting states and superhydrophobic stability of surfaces with dual-scale roughness. Appl. Surf. Sci. 2016, 382, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhang, D.; Lu, Z. Advantage of super-hydrophobic surface as a barrier against atmospheric corrosion induced by salt deliquescence. Corros. Sci. 2015, 90, 23–32. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Wu, J.; Wan, Y. Super-hydrophobic film prepared on zinc and its effect on corrosion in simulated marine atmosphere. Corros. Sci. 2013, 69, 23–30. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Feoktistov, D.V.; Orlova, E.G.; Batishcheva, K.; Ilenok, S. Unification of the textures formed on aluminum after laser treatment. Appl. Surf. Sci. 2019, 469, 974–982. [Google Scholar] [CrossRef]
- Redkina, G.V.; Sergienko, A.S.; Kuznetsov, Y.I. Hydrophobic and anticorrosion properties of thin phosphonate–siloxane films formed on a laser textured zinc surface. Int. J. Corros. Scale Inhib. 2020, 9, 1550–1563. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Sayfutdinova, A.R.; Emelyanenko, K.A.; Vasiliev, A.; Emelyanenko, A. Combination of functional nanoengineering and nanosecond laser texturing for design of superhydrophobic aluminum alloy with exceptional mechanical and chemical properties. ACS Nano 2017, 11, 10113–10123. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, Y.; Zhang, Y.; Lv, Q.; Xu, T.; Liu, T. Super-hydrophobic surface treatment as corrosion protection for aluminum in seawater. Corr. Sci. 2009, 51, 1757–1761. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Zhang, W.; Wu, J.; Yu, X.; Zhang, Y. Stearic acid modified aluminum surfaces with controlled wetting properties and corrosion resistance. Corros. Sci. 2014, 83, 86–93. [Google Scholar] [CrossRef]
- Attavar, S.; Diwekar, M.; Linford, M.R.; Davis, M.A.; Blair, S. Passivation of aluminum with alkyl phosphonic acids for biochip applications. Appl. Surf. Sci. 2010, 256, 7146–7150. [Google Scholar] [CrossRef]
- Hoque, E.; De Rose, J.A.; Hoffmann, P.; Mathieu, H.J.; Bhushan, B.; Cichomski, M. Phosphonate self-assembled monolayers on aluminium surfaces. J. Chem. Phys. 2006, 124, 174710. [Google Scholar] [CrossRef]
- Zhao, R.; Rupper, P.; Gaan, S. Recent development in phosphonic acid-based organic coatings on aluminum. Coatings 2017, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, P.J.; Malicki, M.; Giordano, A.J.; Armstrong, N.R.; Marder, S.R. Characterization of phosphonic acid binding to zinc oxide. J. Mater. Chem. 2011, 21, 3107–3112. [Google Scholar] [CrossRef]
- Hotchkiss, P.J.; Jones, S.C.; Paniagua, S.A.; Sharma, A.; Kippelen, B.; Armstrong, N.; Marder, S. The modification of indium tin oxide with phosphonic acids: Mechanism of binding, tuning of surface properties, and potential for use in organic electronic applications. Acc. Chem. Res. 2012, 45, 337–346. [Google Scholar] [CrossRef]
- Thieme, M.; Worch, H. Ultrahydrophobic aluminium surfaces: Properties and EIS measurements of different oxidic and thin-film coated states. J. Solid State Electrochem. 2006, 10, 737–745. [Google Scholar] [CrossRef]
- Luschtinetz, R.; Oliveira, A.; Duarte, H.A.; Seifert, G. Self-assembled monolayers of alkylphosphonic acids on aluminum oxide surfaces—A theoretical study. J. Inorg. Gen. Chem. 2010, 636, 1506–1512. [Google Scholar] [CrossRef] [Green Version]
- Boyer, Q.; Duluard, S.; Tenailleau, C.; Ansart, F.; Turq, V.; Bonino, J.P. Functionalized superhydrophobic coatings with micro-/nanostructured ZnO particles in a sol–gel matrix. J. Mater. Sci. 2017, 52, 12677–12688. [Google Scholar] [CrossRef]
- Dai, C.; Liu, N.; Cao, Y.; Chen, Y.; Lua, F.; Feng, L. Fast formation of superhydrophobic octadecylphosphonic acid (ODPA) coating for self cleaning and oil/water separation. Soft Matter. 2014, 10, 8116–8121. [Google Scholar] [CrossRef] [PubMed]
- Fontes, G.N.; Malachias, A.; Magalhes-Paniago, R.; Neves, B.R.A. Structural investigations of octadecylphosphonic acid multilayers. Langmuir 2003, 19, 3345–3349. [Google Scholar] [CrossRef]
- Semiletov, A.M.; Chirkunov, A.A.; Kuznetsov, Y.I. Protection of aluminum alloy AD31 from corrosion by adsorption layers of trialkoxysilanes and stearic acid. Mater. Corros. 2020, 71, 77–85. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4713. [Google Scholar] [CrossRef] [Green Version]
- Scofield, H. Hartree-slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley & Sons: Chichester, UK, 1992; 280p. [Google Scholar] [CrossRef]
- Bhure, R.; Abdel-Fattah, T.M.; Bonner, C.; Hall, F.; Mahapatro, A. Stability of phosphonic self assembled monolayers (SAMs) on cobalt chromium (Co–Cr) alloy under oxidative conditions. Appl. Surf. Sci. 2011, 257, 5605–5612. [Google Scholar] [CrossRef] [Green Version]
- Ohno, Y.; Mineo, A.; Matsubara, I. Reflection electron-energy-loss spectroscopy, x-ray-absorption spectroscopy, and x-ray photoelectron spectroscopy studies of a new type of layer compound CrPS4. Phys. Rev. B 1989, 40, 10262–10272. [Google Scholar] [CrossRef]
- Paniagua, S.A.; Hotchkiss, P.J.; Jones, S.C.; Marder, S.R.; Mudalige, A.; Marrikar, F.S.; Armstrong, N.R. Phosphonic acid modification of indium−tin oxide electrodes: Combined XPS/UPS/contact angle studies. J. Phys. Chem. C 2008, 112, 7809–7817. [Google Scholar] [CrossRef]
- Bagus, P.S.; Pacchioni, G.; Parmigiani, F. Surface core-level spectroscopy of Cu(100) and Al(100). Phys. Rev. B 1991, 43, 5172–5175. [Google Scholar] [CrossRef] [PubMed]
- Marcus, P.; Hinnen, C.; Imbert, D.; Siffre, J.M. An in situ XPS study of the formation of aluminium-polymer interfaces. Surf. Interface Anal. 1992, 19, 127–132. [Google Scholar] [CrossRef]
- Gredelj, S.; Gerson, A.R.; Kumar, S.; Cavallaro, G.P. Characterization of aluminium surfaces with and without plasma nitriding by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2001, 174, 240–250. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Gati, D.; Dzhurinskii, B.F.; Sergushin, N.P.; Salyn, Y.V. X-ray electron study of oxides of elements. Zhurnal Neorg. Khimii 1975, 20, 2307–2314. (In Russian) [Google Scholar]








| Treatment | Measurement Parameters | ||||
|---|---|---|---|---|---|
| Rz, μm | Ra, μm | Roughness Class | |||
| After mechanical polishing | 0.46 | 0.04 | 2 | ||
| After etching in 10% NaOH | 1.95 | 0.18 | 4 | ||
| LP mode | Speed, mm/s | Power, W | − | ||
| LP-1 | 300 | 9 | 14.70 | 2.52 | 9 |
| LP-2 | 500 | 9 | 9.82 | 1.82 | 8 |
| LP-3 | 700 | 9 | 9.25 | 1.72 | 8 |
| LP-4 | 500 | 6 | 4.05 | 0.43 | 6 |
| Surface Preparation | Treatment in ODPA | |
|---|---|---|
| − | θc, degree | θc, degree |
| Mechanical polishing | 45 ± 2 |  122 ± 2 |
| Etching in 10% NaOH | 35 ± 2 |  162 ± 2 |
| LP-1 | ≤2 |  162 ± 2 |
| LP-2 | ≤2 |  168 ± 2 |
| LP-3 | ≤2 |  165 ± 2 |
| LP-4 | ≤2 |  161 ± 2 |
| − | − | LT | LT-SA | LT-ODPA |
|---|---|---|---|---|
| Rs, Ω cm2 | − | 745.54 | 963.81 | 595.32 |
| Qf | Y, (S sn cm−2) | − | 7.0009 × 10−9 | 5.4734 × 10−10 |
| n | - | 0.90521 | 0,97652 | |
| Rf, Ωcm2 | − | - | 3.0145 × 105 | 8.734 × 106 |
| Qox | Y(S sn cm−2) | - | 5.0584 × 10−8 | - |
| n | - | 0,66644 | - | |
| Rox, Ω cm2 | − | - | 5.9351 × 106 | - |
| Qdl | Y (S sn cm−2) | 2.7871 × 10−5 | 3.2058 × 10−7 | 5.1606 × 10−9 |
| n | 0.89962 | 0.74578 | 0.62758 | |
| Rp, Ω cm2 | − | 1.3089 × 105 | 6.167 × 107 | 4.0889 × 108 |
| W, (S s1/2) | − | - | - | 3.1653 × 10−8 |
| States | Al0 | Al3+ | ODP-Al |
|---|---|---|---|
| 1 | 1.75 | 2.44 | − |
| 2 | 1.75 | 2.09 | 2.03 |
| States | Al2O3 | Al(OH)3 |
|---|---|---|
| 1 | 9.98 | 90.02 |
| 2 | 18.72 | 81.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semiletov, A.M.; Chirkunov, A.A.; Grafov, O.Y.; Kuznetsov, Y.I. Stability of Supehydrophobic Layers Formed by Organic Acids on the Surface of Aluminum Alloy 6063. Coatings 2022, 12, 1468. https://doi.org/10.3390/coatings12101468
Semiletov AM, Chirkunov AA, Grafov OY, Kuznetsov YI. Stability of Supehydrophobic Layers Formed by Organic Acids on the Surface of Aluminum Alloy 6063. Coatings. 2022; 12(10):1468. https://doi.org/10.3390/coatings12101468
Chicago/Turabian StyleSemiletov, Alexey M., Alexander A. Chirkunov, Oleg Yu. Grafov, and Yurii I. Kuznetsov. 2022. "Stability of Supehydrophobic Layers Formed by Organic Acids on the Surface of Aluminum Alloy 6063" Coatings 12, no. 10: 1468. https://doi.org/10.3390/coatings12101468
APA StyleSemiletov, A. M., Chirkunov, A. A., Grafov, O. Y., & Kuznetsov, Y. I. (2022). Stability of Supehydrophobic Layers Formed by Organic Acids on the Surface of Aluminum Alloy 6063. Coatings, 12(10), 1468. https://doi.org/10.3390/coatings12101468