A Comparative Research on Corrosion Behavior of Electroplated and Magnetron Sputtered Chromium Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cr Thin Films Deposition
2.2. Characterization Methods
3. Results
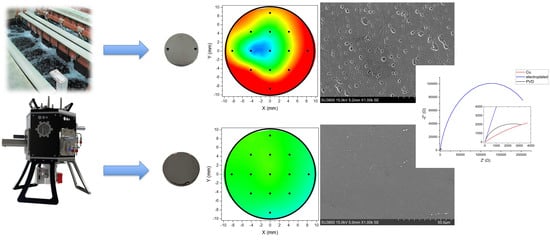
3.1. XRF Thickness Measurement
3.2. Color Measurement
3.3. Morphological and Near Surface Chemical Investigation
3.4. X-ray Investigation
3.5. Electrochemical Characterization
3.5.1. OCP
3.5.2. Potentiodynamic Polarization
3.5.3. Electrochemical Impedance Spectroscopy (EIS)
3.6. Free Corrosion Tests
Salt Spray Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsay, J.H. Decorative and hard chromium plating. Plat. Surf. Finish 1997, 84, 50–51. [Google Scholar]
- Ohmi, T.; Nakagawa, Y.; Nakamura, M.; Ohki, A.; Koyama, T. Formation of chromium oxide on 316L austenitic stainless steel. J. Vac. Sci. Technol. A Vac. Surf. Film. 1996, 14, 2505–2510. [Google Scholar] [CrossRef]
- Durney, L.J. Electroplating Engineering Handbook; Springer: New York, NY, USA, 1996; ISBN 978-0-412-74110-4. [Google Scholar]
- Snyder, D.L. Decorative chromium plating. Met. Finish 2000, 98, 215–222. [Google Scholar] [CrossRef]
- Taylor, S.R. Coatings for Corrosion Protection: Metallic. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1–5. [Google Scholar]
- Schlesinger, M.; Paunovic, M. Modern Electroplating; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 9780470602638. [Google Scholar]
- Wang, Y.; Su, H.; Gu, Y.; Song, X.; Zhao, J. Carcinogenicity of chromium and chemoprevention: A brief update. OncoTargets Ther. 2017, 10, 4065–4079. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Seo, S.; Kim, Y.; Kim, D.H. Review of carcinogenicity of hexavalent chrome and proposal of revising approval standards for an occupational cancers in Korea. Ann. Occup. Environ. Med. 2018, 30, 7. [Google Scholar] [CrossRef] [PubMed]
- Directive 76/769/EEC Restricted substances by Annex XVII to REACH Regulation, Restrictions on the manufacture, placing on the market and use of certain dangerous substances, mixtures and articles. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/HTML/?uri=CELEX:02006R1907-20210215#tocId286 (accessed on 10 February 2022).
- Baral, A.; Engelken, R.D. Chromium-based regulations and greening in metal finishing industries in the USA. Environ. Sci. Policy 2002, 5, 121–133. [Google Scholar] [CrossRef]
- Legg, K.O.; Graham, M.; Chang, P.; Rastagar, F.; Gonzales, A.; Sartwell, B. The replacement of electroplating. Surf. Coat. Technol. 1996, 81, 99–105. [Google Scholar] [CrossRef]
- Del Pianta, D.; Frayret, J.; Gleyzes, C.; Cugnet, C.; Dupin, J.C.; Le Hecho, I. Determination of the chromium(III) reduction mechanism during chromium electroplating. Electrochim. Acta 2018, 284, 234–241. [Google Scholar] [CrossRef]
- Dennis, J.K.; Such, C.E. Nickel and Chromium Plating; Woodhead Publishing: Cambridge, UK, 1993. [Google Scholar]
- Leimbach, M.; Tschaar, C.; Zapf, D.; Kurniawan, M.; Schmidt, U.; Bund, A. Relation between Color and Surface Morphology of Electrodeposited Chromium for Decorative Applications. J. Electrochem. Soc. 2019, 166, D205–D211. [Google Scholar] [CrossRef] [Green Version]
- Leimbach, M.; Tschaar, C.; Schmidt, U.; Bund, A. Low-frequency pulse plating for tailoring the optical appearance of chromium layers for decorative applications. J. Appl. Electrochem. 2020, 50, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, M.; Barrera, E.; Palomar-Pardavé, M.; Huerta, L.; Muhl, S. Characterization of black and white chromium electrodeposition films: Surface and optical properties. J. Non-Cryst. Solids 2003, 329, 31–38. [Google Scholar] [CrossRef]
- Chien, C.W.; Liu, C.L.; Chen, F.J.; Lin, K.H.; Lin, C.S. Microstructure and properties of carbon–sulfur-containing chromium deposits electroplated in trivalent chromium baths with thiosalicylic acid. Electrochim. Acta 2012, 72, 74–80. [Google Scholar] [CrossRef]
- Polukarov, Y.M.; Safonov, V.A.; Edigaryan, A.A.; Vykhodtseva, L.N. Chrome Plating from Sulfate–Oxalate Cr(III) Baths. Structure, Composition, and Corrosion Behavior1. Prot. Met. 2001, 37, 447–451. [Google Scholar] [CrossRef]
- Lansdell, P.; Farr, J.P.G. The Corrosion Resistance of Chromium Electroplated from Trivalent and Hexavalent Chromium Plating Solutions. Trans. IMF 1997, 75, 219–223. [Google Scholar] [CrossRef]
- He, X.; Li, C.; Zhu, Q.; Hou, B.; Jiang, Y.; Wu, L. Electrochemical mechanism of Cr(iii) reduction for preparing crystalline chromium coatings based on 1-butyl-3-methylimidazolium hydrogen sulfate ionic liquid. RSC Adv. 2014, 4, 64174–64182. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Archer, J.; John, C. Electrodeposition of Chromium Black from Ionic Liquids. Trans. IMF 2004, 82, 14–17. [Google Scholar] [CrossRef]
- Verdonck, T.; Verpoort, P.; De Strycker, J.; De Cleene, A.; Banerjee, D.; Nockemann, P.; Van Deun, R.; Van Hecke, K. Chromium(iii) in deep eutectic solvents: Towards a sustainable chromium(vi)-free steel plating process. Green Chem. 2019, 21, 3637–3650. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Zhang, R. Application and Development Progress of Cr-Based Surface Coatings in Nuclear Fuel Element: I. Selection, Preparation, and Characteristics of Coating Materials. Coatings 2020, 10, 808. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.J.G.; Porteiro, J.; Míguez, J.L.; Pinto, G.; Fernandes, L. On the Physical Vapour Deposition (PVD): Evolution of Magnetron Sputtering Processes for Industrial Applications. Procedia Manuf. 2018, 17, 746–757. [Google Scholar] [CrossRef]
- Constantin, R.; Miremad, B. Performance of hard coatings, made by balanced and unbalanced magnetron sputtering, for decorative applications. Surf. Coat. Technol. 1999, 120, 728–733. [Google Scholar] [CrossRef]
- Fenker, M.; Jackson, N.; Spolding, M.; Nicole, P.; Schönhut, K.; Gregory, G.; Hovsepian, P.E.; Münz, W.-D. Corrosion performance of PVD-coated and anodised materials for the decorative market. Surf. Coat. Technol. 2004, 188–189, 466–472. [Google Scholar] [CrossRef]
- Mattox, D.M. The Foundations of Vacuum Coating Technology; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 978-3-662-10329-6. [Google Scholar]
- Martyak, N.M.; McCaskie, J.E.; Voos, B.; Plieth, W. Microcracks in chromium electrodeposits. J. Mater. Sci. 1997, 32, 6069–6073. [Google Scholar] [CrossRef]
- Thornton, J.A.; Hoffman, D.W. Stress-related effects in thin films. Thin Solid Film. 1989, 171, 5–31. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Wasa, K.; Kanno, I.; Kotera, H. (Eds.) Handbook of Sputter Deposition Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Krella, A. Resistance of PVD Coatings to Erosive and Wear Processes: A Review. Coatings 2020, 10, 921. [Google Scholar] [CrossRef]
- Magnetron Sputtering System Market Size, Market Share, Application Analysis, Regional Outlook, Growth Trends, Key Players, Competitive Strategies and Forecasts, 2020 To 2028; Research and Markets, Acute Market Reports. 2021, p. 108. Available online: https://www.researchandmarkets.com/reports/5264911/magnetron-sputtering-system-market-size-market (accessed on 22 December 2021).
- Cialone, M.; Fernandez-Barcia, M.; Celegato, F.; Coisson, M.; Barrera, G.; Uhlemann, M.; Gebert, A.; Sort, J.; Pellicer, E.; Rizzi, P.; et al. A comparative study of the influence of the deposition technique (electrodeposition versus sputtering) on the properties of nanostructured Fe70Pd30 films. Sci. Technol. Adv. Mater. 2020, 21, 424–434. [Google Scholar] [CrossRef]
- Giroire, B.; Ahmad, M.A.; Aubert, G.; Teule-Gay, L.; Michau, D.; Watkins, J.J.; Aymonier, C.; Poulon-Quintin, A. A comparative study of copper thin films deposited using magnetron sputtering and supercritical fluid deposition techniques. Thin Solid Film. 2017, 643, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Carter, E.C.; Schanda, J.D.; Hirschler, R.; Jost, S.; Luo, M.R.; Melgosa, M.; Ohno, Y.; Pointer, M.R.; Rich, D.C.; Viénot, F.; et al. CIE 015:2018-Colorimetry; Wiley Online Library: Vienna, Austria, 2018. [Google Scholar]
- Liang, A.; Zhang, J. Why the decorative chromium coating electrodeposited from trivalent chromium electrolyte containing formic acid is darker. Surf. Coat. Technol. 2012, 206, 3614–3618. [Google Scholar] [CrossRef]
- Anders, A. A structure zone diagram including plasma-based deposition and ion etching. Thin Solid Film. 2010, 518, 4087–4090. [Google Scholar] [CrossRef] [Green Version]
- Sherif, E.M.; Park, S.-M. Inhibition of Copper Corrosion in 3.0% NaCl Solution by N-Phenyl-1,4-phenylenediamine. J. Electrochem. Soc. 2005, 152, B428. [Google Scholar] [CrossRef] [Green Version]
- Moretti, G.; Guidi, F. Tryptophan as copper corrosion inhibitor in 0.5 M aerated sulfuric acid. Corros. Sci. 2002, 44, 1995–2011. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Rajala, P.; Bomberg, M.; Carpén, L. EIS study on aerobic corrosion of copper in ground water: Influence of micro-organisms. Electrochim. Acta 2017, 240, 163–174. [Google Scholar] [CrossRef]









| Samples | L* (D65) | a* (D65) | b* (D65) |
|---|---|---|---|
| ED-Cr | 81.74 | −1.01 | −0.96 |
| MS-Cr | 48.95 | 0.90 | 8.34 |
| Samples | OCP | icorr (A/cm2) | |ba| (V/dec) | |bc| (V/dec) | Polarization Resistance Rct (kΩ) |
|---|---|---|---|---|---|
| Cu | −0.26 ± 0.01 | 2.6 × 10−6 | 0.06 | 0.07 | 13.5 |
| ED-Cr | −0.29 ± 0.01 | 1.6 × 10−7 | 0.06 | 0.15 | 130.7 |
| MS-Cr | −0.02 ± 0.01 | 9.6 × 10−7 | 0.02 | 0.06 | 4.8 |
| Time (h) | 0 | 24 | 48 | 72 | 144 |
|---|---|---|---|---|---|
| ED-Cr |  |  |  |  |  |
| MS-Cr |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinuzzi, S.M.; Donati, L.; Giurlani, W.; Pizzetti, F.; Galvanetto, E.; Calisi, N.; Innocenti, M.; Caporali, S. A Comparative Research on Corrosion Behavior of Electroplated and Magnetron Sputtered Chromium Coatings. Coatings 2022, 12, 257. https://doi.org/10.3390/coatings12020257
Martinuzzi SM, Donati L, Giurlani W, Pizzetti F, Galvanetto E, Calisi N, Innocenti M, Caporali S. A Comparative Research on Corrosion Behavior of Electroplated and Magnetron Sputtered Chromium Coatings. Coatings. 2022; 12(2):257. https://doi.org/10.3390/coatings12020257
Chicago/Turabian StyleMartinuzzi, Stefano Mauro, Lorenzo Donati, Walter Giurlani, Federico Pizzetti, Emanuele Galvanetto, Nicola Calisi, Massimo Innocenti, and Stefano Caporali. 2022. "A Comparative Research on Corrosion Behavior of Electroplated and Magnetron Sputtered Chromium Coatings" Coatings 12, no. 2: 257. https://doi.org/10.3390/coatings12020257
APA StyleMartinuzzi, S. M., Donati, L., Giurlani, W., Pizzetti, F., Galvanetto, E., Calisi, N., Innocenti, M., & Caporali, S. (2022). A Comparative Research on Corrosion Behavior of Electroplated and Magnetron Sputtered Chromium Coatings. Coatings, 12(2), 257. https://doi.org/10.3390/coatings12020257