Designing Gallium-Containing Hydroxyapatite Coatings on Low Modulus Beta Ti-45Nb Alloy
Abstract
:1. Introduction
2. Materials and Methods
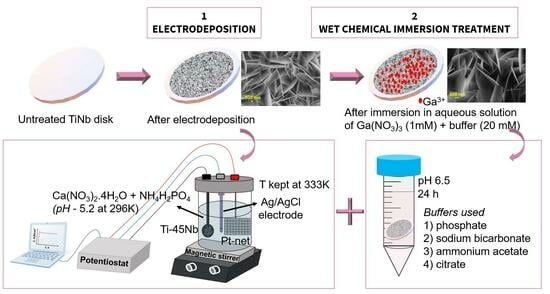
2.1. Electrodeposition of HAP and Ga(NO3)3 Immersion
2.2. Surface Characterization
3. Results and Discussion
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Suwas, S.; Chatterjee, K. Comprehensive review on alloy design, processing, and performance of β Titanium alloys as biomedical materials. Int. Mater. Rev. 2021, 66, 114–139. [Google Scholar] [CrossRef]
- Weng, W.; Biesiekierski, A.; Li, Y.; Wen, C. Effects of selected metallic and interstitial elements on the microstructure and mechanical properties of beta titanium alloys for orthopedic applications. Materialia 2019, 6, 100323. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Martínez-Campos, E.; Mora Sánchez, H.; Moreno, L.; Arrabal, R.; Mohedano, M.; Gallardo, A.; Rodríguez-Hernández, J.; Matykina, E. Hybrid functionalized coatings on Metallic Biomaterials for Tissue Engineering. Surf. Coat. Technol. 2021, 422, 127508. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.; Reis, A.C.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resistance of Biomedical Ti-6Al-4V and β-Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Uskoković, V. Ion-doped hydroxyapatite: An impasse or the road to follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Gadow, R.; Killinger, A.; Stiegler, N. Hydroxyapatite coatings for biomedical applications deposited by different thermal spray techniques. Surf. Coat. Technol. 2010, 205, 1157–1164. [Google Scholar] [CrossRef]
- Paterlini, A.; Alexis, J.; Balcaen, Y.; Bertrand, G. Cold Spraying of Thick Biomimetic and Stoichiometric Apatite Coatings for Orthopaedic Implants. Coatings 2022, 12, 722. [Google Scholar] [CrossRef]
- Teghil, R.; Curcio, M.; De Bonis, A. Substituted Hydroxyapatite, Glass, and Glass-Ceramic Thin Films Deposited by Nanosecond Pulsed Laser Deposition (PLD) for Biomedical Applications: A Systematic Review. Coatings 2021, 11, 811. [Google Scholar] [CrossRef]
- Rabiei, A.; Thomas, B.; Jin, C.; Narayan, R.; Cuomo, J.; Yang, Y.; Ong, J.L. A study on functionally graded HA coatings processed using ion beam assisted deposition with in situ heat treatment. Surf. Coat. Technol. 2006, 200, 6111–6116. [Google Scholar] [CrossRef]
- Schmidt, R.; Hoffmann, V.; Helth, A.; Gostin, P.F.; Calin, M.; Eckert, J.; Gebert, A. Electrochemical deposition of hydroxyapatite on beta-Ti-40Nb. Surf. Coat. Technol. 2016, 294, 186–193. [Google Scholar] [CrossRef]
- Eliaz, N.; Eliyahu, M. Electrochemical processes of nucleation and growth of hydroxyapatite on titanium supported by real-time electrochemical atomic force microscopy. J. Biomed. Mater. Res. Part A 2007, 80A, 621–634. [Google Scholar] [CrossRef]
- Drevet, R.; Ben Jaber, N.; Fauré, J.; Tara, A.; Ben Cheikh Larbi, A.; Benhayoune, H. Electrophoretic deposition (EPD) of nano-hydroxyapatite coatings with improved mechanical properties on prosthetic Ti6Al4V substrates. Surf. Coat. Technol. 2016, 301, 94–99. [Google Scholar] [CrossRef]
- Choudhury, P.; Agrawal, D.C. Sol–gel derived hydroxyapatite coatings on titanium substrates. Surf. Coat. Technol. 2011, 206, 360–365. [Google Scholar] [CrossRef]
- Wen, S.; Liu, X.; Ding, J.; Liu, Y.; Lan, Z.; Zhang, Z.; Chen, G. Hydrothermal synthesis of hydroxyapatite coating on the surface of medical magnesium alloy and its corrosion resistance. Prog. Nat. Sci. Mater. Int. 2021, 31, 324–333. [Google Scholar] [CrossRef]
- Eliaz, N.; Sridhar, T.M. Electrocrystallization of Hydroxyapatite and Its Dependence on Solution Conditions. Cryst. Growth Des. 2008, 8, 3965–3977. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Electrodeposition of Calcium Phosphate Coatings on Metallic Substrates for Bone Implant Applications: A Review. Coatings 2022, 12, 539. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Kurtuldu, F.; Mutlu, N.; Boccaccini, A.R.; Galusek, D. Gallium containing bioactive materials: A review of anticancer, antibacterial, and osteogenic properties. Bioact. Mater. 2022, 17, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Qi, M.; Cheng, S.; Li, C.; Dong, B.; Wang, L. Gallium and gallium compounds: New insights into the “Trojan horse” strategy in medical applications. Mater. Des. 2023, 227, 111704. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium and its competing roles with iron in biological systems. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2044–2053. [Google Scholar] [CrossRef]
- Alberta, L.A.; Vishnu, J.; Hariharan, A.; Pilz, S.; Gebert, A.; Calin, M. Novel low modulus beta-type Ti–Nb alloys by gallium and copper minor additions for antibacterial implant applications. J. Mater. Res. Technol. 2022, 20, 3306–3322. [Google Scholar] [CrossRef]
- Alberta, L.A.; Vishnu, J.; Douest, Y.; Perrin, K.; Trunfio-Sfarghiu, A.-M.; Courtois, N.; Gebert, A.; Ter-Ovanessian, B.; Calin, M. Tribocorrosion behavior of β-type Ti-Nb-Ga alloys in a physiological solution. Tribol. Int. 2023, 181, 108325. [Google Scholar] [CrossRef]
- Alberta, L.A.; Fortouna, Y.; Vishnu, J.; Pilz, S.; Gebert, A.; Lekka, C.; Nielsch, K.; Calin, M. Effects of Ga on the structural, mechanical and electronic properties of β-Ti-45Nb alloy by experiments and ab initio calculations. J. Mech. Behav. Biomed. Mater. 2023, 140, 105728. [Google Scholar] [CrossRef]
- Akman, A.; Alberta, L.A.; Giraldo-Osorno, P.M.; Turner, A.B.; Hantusch, M.; Palmquist, A.; Trobos, M.; Calin, M.; Gebert, A. Effect of minor gallium addition on corrosion, passivity, and antibacterial behaviour of novel β-type Ti–Nb alloys. J. Mater. Res. Technol. 2023, 25, 4110–4124. [Google Scholar] [CrossRef]
- Collery, P.; Keppler, B.; Madoulet, C.; Desoize, B. Gallium in cancer treatment. Crit. Rev. Oncol./Hematol. 2002, 42, 283–296. [Google Scholar] [CrossRef]
- Yang, M.; Ren, J.; Zhang, R. Novel gallium-doped amorphous calcium phosphate nanoparticles: Preparation, application and structure study. J. Non-Cryst. Solids 2017, 466–467, 15–20. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanović, M.; Krajnc, A.; Kramer, L.; Turk, B.; Suvorov, D. Designing Ga(iii)-containing hydroxyapatite with antibacterial activity. RSC Adv. 2016, 6, 112839–112852. [Google Scholar] [CrossRef]
- Mosina, M.; Siverino, C.; Stipniece, L.; Sceglovs, A.; Vasiljevs, R.; Moriarty, T.F.; Locs, J. Gallium-Doped Hydroxyapatite Shows Antibacterial Activity against Pseudomonas aeruginosa without Affecting Cell Metabolic Activity. J. Funct. Biomater. 2023, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Pajor, K.; Pajchel, Ł.; Zgadzaj, A.; Piotrowska, U.; Kolmas, J. Modifications of Hydroxyapatite by Gallium and Silver Ions—Physicochemical Characterization, Cytotoxicity and Antibacterial Evaluation. Int. J. Mol. Sci. 2020, 21, 6. [Google Scholar] [CrossRef]
- Mellier, C.; Fayon, F.; Schnitzler, V.; Deniard, P.; Allix, M.; Quillard, S.; Massiot, D.; Bouler, J.-M.; Bujoli, B.; Janvier, P. Characterization and Properties of Novel Gallium-Doped Calcium Phosphate Ceramics. Inorg. Chem. 2011, 50, 8252–8260. [Google Scholar] [CrossRef]
- Mellier, C.; Schnitzler, V.; Deniard, P.; Bouler, J.M.; Bujoli, B.; Janvier, P. Gallium-Doped β-Tricalcium Phosphate Ceramics: Characterization and Properties. Key Eng. Mater. 2012, 493–494, 195–198. [Google Scholar] [CrossRef]
- Melnikov, P.; de Fatima Cepa Matos, M.; Malzac, A.; Rainho Teixeira, A.; de Albuquerque, D.M. Evaluation of in vitro toxicity of hydroxyapatite doped with gallium. Mater. Lett. 2019, 253, 343–345. [Google Scholar] [CrossRef]
- Verron, E.; Bouler, J.M.; Scimeca, J.C. Gallium as a potential candidate for treatment of osteoporosis. Drug Discov. Today 2012, 17, 1127–1132. [Google Scholar] [CrossRef]
- Benézéth, P.; Diakonov, I.I.; Pokrovski, G.S.; Dandurand, J.-L.; Schott, J.; Khodakovsky, I.L. Gallium speciation in aqueous solution. Experimental study and modelling: Part 2. Solubility of α-GaOOH in acidic solutions from 150 to 250 °C and hydrolysis constants of gallium (III) to 300 °C. Geochim. Cosmochim. Acta 1997, 61, 1345–1357. [Google Scholar] [CrossRef]
- Schmidt, R.; Gebert, A.; Schumacher, M.; Hoffmann, V.; Voss, A.; Pilz, S.; Uhlemann, M.; Lode, A.; Gelinsky, M. Electrodeposition of Sr-substituted hydroxyapatite on low modulus beta-type Ti-45Nb and effect on in vitro Sr release and cell response. Mater. Sci. Eng. C 2020, 108, 110425. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Gebert, A.; Oswald, S.; Helth, A.; Voss, A.; Gostin, P.F.; Rohnke, M.; Janek, J.; Calin, M.; Eckert, J. Effect of indium (In) on corrosion and passivity of a beta-type Ti–Nb alloy in Ringer’s solution. Appl. Surf. Sci. 2015, 335, 213–222. [Google Scholar] [CrossRef]
- Kuo, M.C.; Yen, S.K. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mater. Sci. Eng. C 2002, 20, 153–160. [Google Scholar] [CrossRef]
- Bonchi, C.; Imperi, F.; Minandri, F.; Visca, P.; Frangipani, E. Repurposing of gallium-based drugs for antibacterial therapy. BioFactors 2014, 40, 303–312. [Google Scholar] [CrossRef]
- Uskoković, V. The role of hydroxyl channel in defining selected physicochemical peculiarities exhibited by hydroxyapatite. RSC Adv. 2015, 5, 36614–36633. [Google Scholar] [CrossRef]
- López-Ortiz, S.; Mendoza-Anaya, D.; Sánchez-Campos, D.; Fernandez-García, M.E.; Salinas-Rodríguez, E.; Reyes-Valderrama, M.I.; Rodríguez-Lugo, V. The pH Effect on the Growth of Hexagonal and Monoclinic Hydroxyapatite Synthesized by the Hydrothermal Method. J. Nanomater. 2020, 2020, 5912592. [Google Scholar] [CrossRef]







| Sample Code | Buffer Used | Chemical Formula | Concentration | pH Range | Provider |
|---|---|---|---|---|---|
| Gaphos | phosphate | (I) KH2PO4/ | 20 mM | 6.5–6.6 | Merck Millipore, Darmstadt, Germany |
| (II) Na2HPO4 | |||||
| Gacarb | sodium bicarbonate | NaHCO3 | 20 mM | 6.8–6.9 | Merck Millipore, Darmstadt, Germany |
| Gaacet | ammonium acetate | CH3COONH4 | 20 mM | 6.6–6.7 | Merck Millipore, Darmstadt, Germany |
| Gacitr | citrate | HOC(CO2H)(CH2CO2H)2 | 20 mM | 6.5–6.6 | VWR Chemicals, Leuven, Belgium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnu, J.; Voss, A.; Hoffmann, V.; Alberta, L.A.; Akman, A.; Shankar, B.; Gebert, A.; Calin, M. Designing Gallium-Containing Hydroxyapatite Coatings on Low Modulus Beta Ti-45Nb Alloy. Coatings 2023, 13, 1817. https://doi.org/10.3390/coatings13101817
Vishnu J, Voss A, Hoffmann V, Alberta LA, Akman A, Shankar B, Gebert A, Calin M. Designing Gallium-Containing Hydroxyapatite Coatings on Low Modulus Beta Ti-45Nb Alloy. Coatings. 2023; 13(10):1817. https://doi.org/10.3390/coatings13101817
Chicago/Turabian StyleVishnu, Jithin, Andrea Voss, Volker Hoffmann, Ludovico Andrea Alberta, Adnan Akman, Balakrishnan Shankar, Annett Gebert, and Mariana Calin. 2023. "Designing Gallium-Containing Hydroxyapatite Coatings on Low Modulus Beta Ti-45Nb Alloy" Coatings 13, no. 10: 1817. https://doi.org/10.3390/coatings13101817
APA StyleVishnu, J., Voss, A., Hoffmann, V., Alberta, L. A., Akman, A., Shankar, B., Gebert, A., & Calin, M. (2023). Designing Gallium-Containing Hydroxyapatite Coatings on Low Modulus Beta Ti-45Nb Alloy. Coatings, 13(10), 1817. https://doi.org/10.3390/coatings13101817