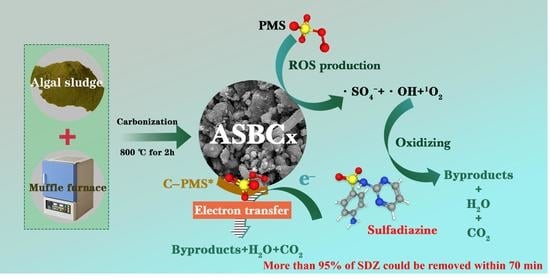
N-Rich Algal Sludge Biochar for Peroxymonosulfate Activation toward Sulfadiazine Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Regents
2.2. Preparation and Characterization of the ASBCx
2.3. Adsorption Studies
2.4. Catalytic Degradation of SDZ
3. Results and Discussion
3.1. Characterizations
3.2. Adsorptive Experiment
3.3. Catalytic Degradation of SDZ
3.4. Stability of the ASBC800/PMS System
3.5. Activation Mechanism
3.5.1. Identification of Reactive Oxygen Species
3.5.2. Electrochemical Analysis
3.6. Durability and Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, J.; Zhu, Y.; Lang, J.; Zhang, J.; Cheng, S.; Zhou, B.; Zhang, L.; Alvarez, P.; Long, M. Spin-State-Dependent Peroxymonosulfate Activation of Single-Atom M–N Moieties via a Radical-Free Pathway. ACS Catal. 2021, 11, 9569–9577. [Google Scholar] [CrossRef]
- Xiao, C.; Hu, Y.; Li, Q.; Liu, J.; Li, X.; Shi, Y.; Chen, Y.; Cheng, J. Carbon-doped defect MoS2 co-catalytic Fe3+/peroxymonosulfate process for efficient sulfadiazine degradation: Accelerating Fe3+/Fe2+ cycle and 1O2 dominated oxidation. Sci. Total Environ. 2023, 858, 159587. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, S.; Huang, J.; Jiang, H. Oxygen-doped biochar for the activation of ferrate for the highly efficient degradation of sulfadiazine with a distinct pathway. J. Environ. Chem. Eng. 2022, 10, 108537. [Google Scholar] [CrossRef]
- Yu, J.; Feng, H.; Tang, L.; Pang, Y.; Zeng, G.; Lu, Y.; Dong, H.; Wang, J.; Liu, Y.; Feng, C.; et al. Metal-free carbon materials for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Prog. Mater. Sci. 2020, 111, 100654. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, H. Direct Electron-Transfer-Based Peroxymonosulfate Activation by Iron-Doped Manganese Oxide (delta-MnO2) and the Development of Galvanic Oxidation Processes (GOPs). Environ. Sci. Technol. 2019, 53, 12610–12620. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Su, H.; Chen, Z.; Yu, C.; Li, Q.; Zhou, B.; Alvarez, P.; Long, M. Selective Degradation of Organic Pollutants Using an Efficient Metal-Free Catalyst Derived from Carbonized Polypyrrole via Peroxymonosulfate Activation. Environ. Sci. Technol. 2017, 51, 11288–11296. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Geng, W.; Alvarez, P.; Long, M. 2D N-Doped Porous Carbon Derived from Polydopamine-Coated Graphitic Carbon Nitride for Efficient Nonradical Activation of Peroxymonosulfate. Environ. Sci. Technol. 2020, 54, 8473–8481. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, S. Metal-Free Carbocatalysis in Advanced Oxidation Reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef]
- Cheng, X.; Li, P.; Zhou, W.; Wu, D.; Luo, C.; Liu, W.; Ren, Z.; Liang, H. Effect of peroxymonosulfate oxidation activated by powdered activated carbon for mitigating ultrafiltration membrane fouling caused by different natural organic matter fractions. Chemosphere 2019, 221, 812–823. [Google Scholar] [CrossRef]
- Fu, C.; Sun, G.; Wang, C.; Wei, B.; Ran, G.; Song, Q. Fabrication of nitrogen-doped graphene nanosheets anchored with carbon nanotubes for the degradation of tetracycline in saline water. Environ. Res. 2022, 206, 112242. [Google Scholar] [CrossRef]
- Huo, X.; Zhou, P.; Zhang, J.; Liu, Y.; Cheng, X.; Liu, Y.; Li, W.; Zhang, Y. N, S-Doped porous carbons for persulfate activation to remove tetracycline: Nonradical mechanism. J. Hazard. Mater. 2020, 391, 122055. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, Z.; He, S.; Hao, F.; Yang, Y.; Lv, Y.; Zhang, W.; Liu, P.; Luo, H. Nitrogen-doped carbon nanotubes as a highly active metal-free catalyst for nitrobenzene hydrogenation. Appl. Catal. B Environ. 2020, 260, 118105. [Google Scholar] [CrossRef]
- Yang, B.; Kang, H.; Ko, Y.; Woo, H.; Gim, G.; Choi, J.; Kim, J.; Cho, K.; Kim, E.; Lee, S.; et al. Persulfate activation by nanodiamond-derived carbon onions: Effect of phase transformation of the inner diamond core on reaction kinetics and mechanisms. Appl. Catal. B Environ. 2021, 293, 120205. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q.; et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer. Appl. Catal. B Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Ho, S.; Zhou, Y.; Ren, N. Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature. Bioresour. Technol. 2018, 259, 104–110. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Feng, H.; Zou, J.; Wang, J.; Feng, C.; Zhu, X.; Ouyang, X.; et al. Hierarchical porous biochar from shrimp shell for persulfate activation: A two-electron transfer path and key impact factors. Appl. Catal. B Environ. 2020, 260, 118160. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Si, Q.; Luo, H.; Zhao, Q.; Ren, N. Sludge-derived biochar as efficient persulfate activators: Sulfurization-induced electronic structure modulation and disparate nonradical mechanisms. Appl. Catal. B Environ. 2020, 279, 119361. [Google Scholar] [CrossRef]
- Mian, M.; Liu, G.; Fu, B.; Song, Y. Facile synthesis of sludge-derived MnOx-N-biochar as an efficient catalyst for peroxymonosulfate activation. Appl. Catal. B Environ. 2019, 255, 117765. [Google Scholar] [CrossRef]
- Zaeni, J.; Lim, W.; Wang, Z.; Ding, D.; Chua, Y.; Ng, S.; Oh, W. In situ nitrogen functionalization of biochar via one-pot synthesis for catalytic peroxymonosulfate activation: Characteristics and performance studies. Sep. Purif. Technol. 2020, 241, 116702. [Google Scholar] [CrossRef]
- Mian, M.; Liu, G.; Zhou, H. Preparation of N-doped biochar from sewage sludge and melamine for peroxymonosulfate activation: N-functionality and catalytic mechanisms. Sci. Total Environ. 2020, 744, 140862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, Y.; Duan, X.; Liu, Y.; Wang, S. Density Functional Theory Calculations for Insight into the Heterocatalyst Reactivity and Mechanism in Persulfate-Based Advanced Oxidation Reactions. ACS Catal. 2021, 11, 11129–11159. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Liu, H.; Liu, H.; Tao, J.; Cui, M.; Zheng, Z.; Wen, D.; Zhan, X. FexN produced in pharmaceutical sludge biochar by endogenous Fe and exogenous N doping to enhance peroxymonosulfate activation for levofloxacin degradation. Water Res. 2022, 224, 119022. [Google Scholar] [CrossRef]
- Ren, F.; Zhu, W.; Zhao, J.; Liu, H.; Zhang, X.; Zhang, H.; Zhu, H.; Peng, Y.; Wang, B. Nitrogen-doped graphene oxide aerogel anchored with spinel CoFe2O4 nanoparticles for rapid degradation of tetracycline. Sep. Purif. Technol. 2020, 241, 116690. [Google Scholar] [CrossRef]
- Ouasfi, N.; Zbair, M.; Bouzikri, S.; Anfar, Z.; Bensitel, M.; Ait Ahsaine, H.; Sabbar, E.; Khamliche, L. Selected pharmaceuticals removal using algae derived porous carbon: Experimental, modeling and DFT theoretical insights. RSC Adv. 2019, 9, 9792–9808. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Wu, Y.; Bian, Y.; Song, Y.; Sun, Q.; Jiang, X.; Zhang, L.; Han, J.; Cheng, H. Nitrogen-doped porous biochar derived from marine algae for efficient solid-phase microextraction of chlorobenzenes from aqueous solution. J. Hazard. Mater. 2021, 407, 124785. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Zhao, H.; Yan, Q. Adsorption and Fenton-like removal of chelated nickel from Zn-Ni alloy electroplating wastewater using activated biochar composite derived from Taihu blue algae. Chem. Eng. J. 2020, 379, 122372. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liu, G.; Yan, Q. In-situ pyrolysis of Taihu blue algae biomass as appealing porous carbon adsorbent for CO2 capture: Role of the intrinsic N. Sci. Total Environ. 2021, 771, 145424. [Google Scholar] [CrossRef]
- Gaballah, M.; Guo, J.; Hassanein, A.; Sobhi, M.; Zheng, Y.; Philbert, M.; Li, B.; Sun, H.; Dong, R. Removal performance and inhibitory effects of combined tetracycline, oxytetracycline, sulfadiazine, and norfloxacin on anaerobic digestion process treating swine manure. Sci. Total Environ. 2023, 857, 159536. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Wang, J.; Deng, Y.; Liu, Y.; Feng, H.; Chen, S.; Ren, X. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism. Chem. Eng. J. 2019, 364, 146–159. [Google Scholar] [CrossRef]
- Zhong, K.; Li, M.; Yang, Y.; Zhang, H.; Zhang, B.; Tang, J.; Yan, J.; Su, M.; Yang, Z. Nitrogen-doped biochar derived from watermelon rind as oxygen reduction catalyst in air cathode microbial fuel cells. Appl. Energ. 2019, 242, 516–525. [Google Scholar] [CrossRef]
- Zhu, K.; Bin, Q.; Shen, Y.; Huang, J.; He, D.; Chen, W. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants. Chem. Eng. J. 2020, 402, 126090. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Zhou, M.; Su, X.; Sun, J. High-efficiency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation. J. Hazard. Mater. 2020, 397, 122764. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Dou, S.; Qin, G.; Wang, B.; Yan, P.; Isimjan, T.; Yang, X. Rational design of highly selective nitrogen-doped Fe2O3-CNTs catalyst towards H2O2 generation in alkaline media. Int. J. Hydrogen Energ. 2020, 45, 6128–6137. [Google Scholar] [CrossRef]
- Qi, Y.; Ge, B.; Zhang, Y.; Jiang, B.; Wang, C.; Akram, M.; Xu, X. Three-dimensional porous graphene-like biochar derived from Enteromorpha as a persulfate activator for sulfamethoxazole degradation: Role of graphitic N and radicals transformation. J. Hazard. Mater. 2020, 399, 123039. [Google Scholar] [CrossRef]
- Ho, S.; Chen, Y.; Li, R.; Zhang, C.; Ge, Y.; Cao, G.; Ma, M.; Duan, X.; Wang, S.; Ren, N. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019, 159, 77–86. [Google Scholar] [CrossRef]
- Dong, F.; Yan, L.; Huang, S.; Liang, J.; Zhang, W.; Yao, X.; Chen, X.; Qian, W.; Guo, P.; Kong, L.; et al. Removal of antibiotics sulfadiazine by a biochar based material activated persulfate oxidation system: Performance, products and mechanism. Process Saf. Environ. 2021, 157, 411–419. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, D.; Deng, Y.; Zhang, T.; Shih, K. Sulfate Radical-Mediated Degradation of Sulfadiazine by CuFeO2 Rhombohedral Crystal-Catalyzed Peroxymonosulfate: Synergistic Effects and Mechanisms. Environ. Sci. Technol. 2016, 50, 3119–3127. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Y.; Zhou, Y.; Liu, X.; Wang, C.; Lan, Y.; Li, Y. Co3O4-MnO2 nanoparticles moored on biochar as a catalyst for activation of peroxymonosulfate to efficiently degrade sulfonamide antibiotics. Sep. Purif. Technol. 2021, 281, 119935. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Yang, B.; Yang, Z.; Shih, K. Activation of peroxymonosulfate by molybdenum disulfide-mediated traces of Fe(III) for sulfadiazine degradation. Chemosphere 2021, 283, 131212. [Google Scholar] [CrossRef]
- Liu, T.; Wu, K.; Wang, M.; Jing, C.; Chen, Y.; Yang, S.; Jin, P. Performance and mechanisms of sulfadiazine removal using persulfate activated by Fe3O4@CuOx hollow spheres. Chemosphere 2021, 262, 127845. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Lu, X.; Cui, X.; Jian, X.; Hu, Z.; Dong, Y.; Liu, X.; Huang, J.; Deng, L. Novel activation of peroxymonosulfate by an easily recyclable VC@Fe3O4 nanoparticles for enhanced degradation of sulfadiazine. Chem. Eng. J. 2019, 363, 318–328. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, H.; Deng, L.; Shi, Z. Peroxymonosulfate-assisted photocatalytic degradation of sulfadiazine using self-assembled multi-layered CoAl-LDH/g-C3N4 heterostructures: Performance, mechanism and eco-toxicity evaluation. J. Water Process Eng. 2019, 33, 101084. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, Z.; Deng, L.; Duan, Y. Efficient degradation of sulfadiazine using magnetically recoverable MnFe2O4/δ-MnO2 hybrid as a heterogeneous catalyst of peroxymonosulfate. Colloid Surface A 2021, 609, 125637. [Google Scholar] [CrossRef]










| Samples | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| ASBC400 | 84.017 | 0.194 | 9.756 |
| ASBC500 | 93.210 | 0.188 | 7.447 |
| ASBC600 | 110.686 | 0.232 | 7.636 |
| ASBC800 | 145.596 | 0.179 | 6.220 |
| Biochar | Pseudo First-Order | Pseudo Second-Order | ||||
|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k2 | R2 | |
| ASBC400 | 0.461 | 0.721 | 0.941 | 0.482 | 2.696 | 0.967 |
| ASBC500 | 0.915 | 0.239 | 0.912 | 0.993 | 0.435 | 0.969 |
| ASBC600 | 1.144 | 0.881 | 0.991 | 1.175 | 1.816 | 0.999 |
| ASBC800 | 11.763 | 0.352 | 0.985 | 12.532 | 0.043 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Chen, Z.; Kang, R.; Wang, J.; Lu, Q.; Wang, T.; Tian, D.; Xu, Y.; Wang, Z.; Ding, H. N-Rich Algal Sludge Biochar for Peroxymonosulfate Activation toward Sulfadiazine Removal. Coatings 2023, 13, 431. https://doi.org/10.3390/coatings13020431
Liu C, Chen Z, Kang R, Wang J, Lu Q, Wang T, Tian D, Xu Y, Wang Z, Ding H. N-Rich Algal Sludge Biochar for Peroxymonosulfate Activation toward Sulfadiazine Removal. Coatings. 2023; 13(2):431. https://doi.org/10.3390/coatings13020431
Chicago/Turabian StyleLiu, Chao, Zhenxiang Chen, Ruiqin Kang, Jing Wang, Qingwei Lu, Tao Wang, Dayong Tian, Ying Xu, Zhan Wang, and Huiping Ding. 2023. "N-Rich Algal Sludge Biochar for Peroxymonosulfate Activation toward Sulfadiazine Removal" Coatings 13, no. 2: 431. https://doi.org/10.3390/coatings13020431
APA StyleLiu, C., Chen, Z., Kang, R., Wang, J., Lu, Q., Wang, T., Tian, D., Xu, Y., Wang, Z., & Ding, H. (2023). N-Rich Algal Sludge Biochar for Peroxymonosulfate Activation toward Sulfadiazine Removal. Coatings, 13(2), 431. https://doi.org/10.3390/coatings13020431