pH-Sensitive Fluorescence Emission of Boron/Nitrogen Co-Doped Carbon Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of B/N-CQDs via Hydrothermal Synthesis
2.2. Characterization of B/N-CQDs
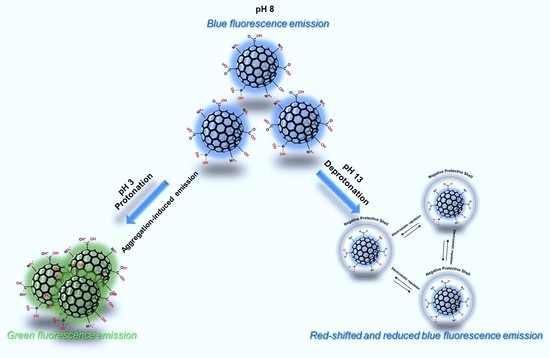
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aydin, S.; Ustun, O.; Ghosigharehaghaji, A.; Tavaci, T.; Yilmaz, A.; Yilmaz, M. Hydrothermal Synthesis of Nitrogen-Doped and Excitation-Dependent Carbon Quantum Dots for Selective Detection of Fe3+ in Blood Plasma. Coatings 2022, 12, 1311. [Google Scholar] [CrossRef]
- John, B.K.; Abraham, T.; Mathew, B. A Review on Characterization Techniques for Carbon Quantum Dots and Their Applications in Agrochemical Residue Detection. J. Fluoresc. 2022, 32, 449–471. [Google Scholar] [CrossRef]
- Karadag, S.N.; Ustun, O.; Yilmaz, A.; Yilmaz, M. The Fabrication of Excitation-Dependent Fluorescence Boron/Nitrogen Co-Doped Carbon Quantum Dots and Their Employment in Bioimaging. Chem. Phys. 2022, 562, 111678. [Google Scholar] [CrossRef]
- Malavika, J.P.; Shobana, C.; Sundarraj, S.; Ganeshbabu, M.; Kumar, P.; Selvan, R.K. Green Synthesis of Multifunctional Carbon Quantum Dots: An Approach in Cancer Theranostics. Biomater. Adv. 2022, 136, 212756. [Google Scholar] [CrossRef]
- Hu, X.; Han, W.; Zhang, M.; Li, D.; Sun, H. Enhanced Adsorption and Visible-Light Photocatalysis on TiO2 with in situ Formed Carbon Quantum Dots. Environ. Sci. Pollut. Res. 2022, 29, 56379–56392. [Google Scholar] [CrossRef]
- Dhandapani, E.; Sengodan, P.; Duraisamy, N.; Ramesh, R. Fabrication of Semi-Flexible Carbon Quantum Dots-Reinforced Polypyrrole (PPy/CQDs) Composite Electrodes by Hybrid Electrospray Deposition for High-Performance Energy Storage Device. Int. J. Energy Res. 2022, 46, 7277–7292. [Google Scholar] [CrossRef]
- Behi, M.; Gholami, L.; Naficy, S.; Palomba, S.; Dehghani, F. Carbon Dots: A Novel Platform for Biomedical Applications. Nanoscale Adv. 2022, 4, 353–376. [Google Scholar] [CrossRef]
- Eivazzadeh-keihan, R.; Noruzi, E.B.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Gorab, M.G.; Hashemi, S.M.; Javanshir, S.; Cohan, R.A.; et al. Applications of Carbon-Based Conductive Nanomaterials in Biosensors; Elsevier B.V.: Amsterdam, The Netherlands, 2022; ISBN 9821732283. [Google Scholar]
- Şen, F.B.; Beğiç, N.; Bener, M.; Apak, R. Fluorescence Turn-off Sensing of TNT by Polyethylenimine Capped Carbon Quantum Dots. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2022, 271, 120884. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, F.; Hu, J.; Gao, W.; Zhang, M. A Mini Review on PH-Sensitive Photoluminescence in Carbon Nanodots. Front. Chem. 2021, 8, 605028. [Google Scholar] [CrossRef]
- Ding, H.; Li, X.H.; Chen, X.B.; Wei, J.S.; Li, X.B.; Xiong, H.M. Surface States of Carbon Dots and Their Influences on Luminescence. J. Appl. Phys. 2020, 127, 231101. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The Photoluminescence Mechanism in Carbon Dots (Graphene Quantum Dots, Carbon Nanodots, and Polymer Dots): Current State and Future Perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Hallaji, Z.; Najafi Nobar, S.; Bagheri, Z. Carbon Dots with PH-Responsive Fluorescence: A Review on Synthesis and Cell Biological Applications. Microchim. Acta 2020, 187, 150. [Google Scholar] [CrossRef]
- Rahmani, E.; Pourmadadi, M.; Ghorbanian, S.A.; Yazdian, F.; Rashedi, H.; Navaee, M. Preparation of a PH-Responsive Chitosan-Montmorillonite-Nitrogen-Doped Carbon Quantum Dots Nanocarrier for Attenuating Doxorubicin Limitations in Cancer Therapy. Eng. Life Sci. 2022, 22, 634–649. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Xiao, N.; Liu, S.G.; Han, L.; Li, N.B.; Luo, H.Q. PH-Induced Aggregation of Hydrophilic Carbon Dots for Fluorescence Detection of Acidic Amino Acid and Intracellular PH Imaging. Mater. Sci. Eng. C 2020, 108, 110401. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Hu, J.; Wei, H.; Liu, X.; Li, E.; Yang, S. Rational Design of Novel Carbon-Oxygen Quantum Dots for Ratiometrically Mapping PH and Reactive Oxygen Species Scavenging. Carbon N. Y. 2022, 190, 115–124. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent Carbon Nanoparticles Derived from Candle Soot. Angew. Chemie 2007, 119, 6593–6595. [Google Scholar] [CrossRef]
- Lu, S.; Cong, R.; Zhu, S.; Zhao, X.; Liu, J.; Tse, J.S.; Meng, S.; Yang, B. PH-Dependent Synthesis of Novel Structure-Controllable Polymer-Carbon NanoDots with High Acidophilic Luminescence and Super Carbon Dots Assembly for White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2016, 8, 4062–4068. [Google Scholar] [CrossRef]
- Zhu, J.; Chu, H.; Shen, J.; Wang, C.; Wei, Y. Carbon Quantum Dots with PH-Responsive Orange-/Red-Light Emission for Fluorescence Imaging of Intracellular PH. Microchim. Acta 2023, 190, 21. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Li, B.; Shen, C.; Sun, Z.; Zhan, Y.; Zhang, Y. Highly Luminescent PH-Responsive Carbon Quantum Dots for Cell Imaging. Nanotechnology 2022, 33, 265002. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Zhang, W.; Qin, X.; Zhu, C. Carbon Quantum Dots Derived from Pure Solvent Tetrahydrofuran as a Fluorescent Probe to Detect PH and Silver Ion. J. Photochem. Photobiol. A Chem. 2019, 382, 111981. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Li, W.; Han, Y.; Liu, Y.; Li, Z.; Zhang, B.; Pan, D. Rationally Designed Efficient Dual-Mode Colorimetric/Fluorescence Sensor Based on Carbon Dots for Detection of PH and Cu2+ Ions. ACS Sustain. Chem. Eng. 2018, 6, 12668–12674. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Wang, C.; Tong, D.; Wu, Q.; Jiang, K.; Jiang, Y.; Wang, C.; Yang, M. Red Emitting and Highly Stable Carbon Dots with Dual Response to PH Values and Ferric Ions. Microchim. Acta 2018, 185, 83. [Google Scholar] [CrossRef]
- Ye, X.; Xiang, Y.; Wang, Q.; Li, Z.; Liu, Z. A Red Emissive Two-Photon Fluorescence Probe Based on Carbon Dots for Intracellular PH Detection. Small 2019, 15, e1901673. [Google Scholar] [CrossRef]
- Choi, Y.; Kang, B.; Lee, J.; Kim, S.; Kim, G.T.; Kang, H.; Lee, B.R.; Kim, H.; Shim, S.H.; Lee, G.; et al. Integrative Approach toward Uncovering the Origin of Photoluminescence in Dual Heteroatom-Doped Carbon Nanodots. Chem. Mater. 2016, 28, 6840–6847. [Google Scholar] [CrossRef]
- Das, S.K.; Liu, Y.; Yeom, S.; Kim, D.Y.; Richards, C.I. Single-Particle Fluorescence Intensity Fluctuations of Carbon Nanodots. Nano Lett. 2014, 14, 620–625. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.C.; Huang, H.; Feng, J.J.; Chen, J.R.; Wang, A.J. Facile Synthesis of Water-Soluble and Biocompatible Fluorescent Nitrogen-Doped Carbon Dots for Cell Imaging. Analyst 2014, 139, 1692–1696. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Cong, S.; Wu, J.; Gao, L.; Wang, Y.; Dai, X.; Yi, Q.; Zou, G. Nitrogen-Doped Carbon Dots for “Green” Quantum Dot Solar Cells. Nanoscale Res. Lett. 2016, 11, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Richardson, I.E.G. Front Matter and Index. In Video Codec Design; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. i–x. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; ISBN 0387312781. [Google Scholar]
- Ye, R.; Xiang, C.; Lin, J.; Peng, Z.; Huang, K.; Yan, Z.; Cook, N.P.; Samuel, E.L.G.; Hwang, C.C.; Ruan, G.; et al. Coal as an Abundant Source of Graphene Quantum Dots. Nat. Commun. 2013, 4, 2943. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Yang, X.; Li, J.; Li, D.; Wang, E. Stable Cu Nanoclusters: From an Aggregation-Induced Emission Mechanism to Biosensing and Catalytic Applications. Chem. Commun. 2014, 50, 237–239. [Google Scholar] [CrossRef]
- Su, Y.; Xie, Z.; Zheng, M. Carbon Dots with Concentration-Modulated Fluorescence: Aggregation-Induced Multicolor Emission. J. Colloid Interface Sci. 2020, 573, 241–249. [Google Scholar] [CrossRef]
- Keerthana, P.; Cherian, A.R.; Sirimahachai, U.; Thadathil, D.A.; Varghese, A.; Hegde, G. Detection of Picric Acid in Industrial Effluents Using Multifunctional Green Fluorescent B/N-Carbon Quantum Dots. J. Environ. Chem. Eng. 2022, 10, 107209. [Google Scholar] [CrossRef]
- Mohammed, L.J.; Omer, K.M. Dual Functional Highly Luminescence B, N Co-Doped Carbon Nanodots as Nanothermometer and Fe3+/Fe2+ Sensor. Sci. Rep. 2020, 10, 3028. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Chen, L.; Wang, J.; Liu, X.; Yang, Y.; Yu, S. Investigation on the Chirality Mechanism of Chiral Carbon Quantum Dots Derived from Tryptophan. RSC Adv. 2019, 9, 3208–3214. [Google Scholar] [CrossRef] [Green Version]
- Mutuma, B.K.; Matsoso, B.J.; Momodu, D.; Oyedotun, K.O.; Coville, N.J.; Manyala, N. Deciphering the Structural, Textural, and Electrochemical Properties of Activated BN-Doped Spherical Carbons. Nanomaterials 2019, 9, 446. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Song, X.; Wang, Y.; Hu, Z.; Yan, F.; Feng, G. Developed a Ratiometric Fluorescence PH Nanosensor Based on Label-Free Carbon Dots for Intracellular Lysosome Imaging and Water PH Monitoring with a Smartphone. Dye. Pigment. 2021, 193, 109490. [Google Scholar] [CrossRef]
- Song, Z.; Quan, F.; Xu, Y.; Liu, M.; Cui, L.; Liu, J. Multifunctional N,S Co-Doped Carbon Quantum Dots with PH- and Thermo-Dependent Switchable Fluorescent Properties and Highly Selective Detection of Glutathione. Carbon N. Y. 2016, 104, 169–178. [Google Scholar] [CrossRef]
- Lei, C.W.; Hsieh, M.L.; Liu, W.R. A Facile Approach to Synthesize Carbon Quantum Dots with PH-Dependent Properties. Dye. Pigment. 2019, 169, 73–80. [Google Scholar] [CrossRef]
- Yu, J.; Liu, C.; Yuan, K.; Lu, Z.; Cheng, Y.; Li, L.; Zhang, X.; Jin, P.; Meng, F.; Liu, H. Luminescence Mechanism of Carbon Dots by Tailoring Functional Groups for Sensing Fe3+ Ions. Nanomaterials 2018, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Wang, H.; Qian, Z.; Yi, Q.; Wang, Y.; Cong, S.; Zhao, J.; Sun, Y.; Huang, J.; Xiong, J.; et al. Emission Switching in Carbon Dots Coated CdTe Quantum Dots Driving by PH Dependent Hetero-Interactions. Appl. Phys. Lett. 2015, 107, 203108. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ustun, O.; Karadag, S.N.; Mazlumoglu, H.; Yilmaz, A.; Yilmaz, M. pH-Sensitive Fluorescence Emission of Boron/Nitrogen Co-Doped Carbon Quantum Dots. Coatings 2023, 13, 456. https://doi.org/10.3390/coatings13020456
Ustun O, Karadag SN, Mazlumoglu H, Yilmaz A, Yilmaz M. pH-Sensitive Fluorescence Emission of Boron/Nitrogen Co-Doped Carbon Quantum Dots. Coatings. 2023; 13(2):456. https://doi.org/10.3390/coatings13020456
Chicago/Turabian StyleUstun, Oguzhan, Sugra Naz Karadag, Hayrunnisa Mazlumoglu, Asli Yilmaz, and Mehmet Yilmaz. 2023. "pH-Sensitive Fluorescence Emission of Boron/Nitrogen Co-Doped Carbon Quantum Dots" Coatings 13, no. 2: 456. https://doi.org/10.3390/coatings13020456
APA StyleUstun, O., Karadag, S. N., Mazlumoglu, H., Yilmaz, A., & Yilmaz, M. (2023). pH-Sensitive Fluorescence Emission of Boron/Nitrogen Co-Doped Carbon Quantum Dots. Coatings, 13(2), 456. https://doi.org/10.3390/coatings13020456