Assessing the Bioreceptivity of Biobased Cladding Materials
Abstract
:1. Introduction
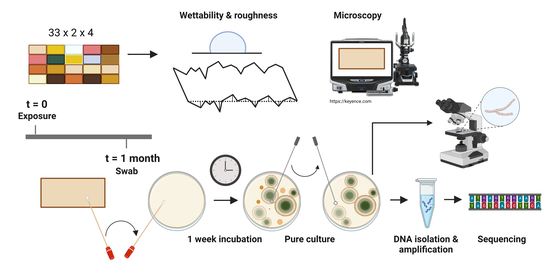
2. Materials and Methods
2.1. Experimental Materials and Natural Weathering
2.2. Surface Wettability and Topography Measurements
2.3. Evaluation of Microbial Growth
2.4. Visualisation and Identification of Fungal Macro- and Micromorphological Features
2.5. Molecular Identification of Selected Isolates
2.6. Data Analysis
3. Results and Discussion
3.1. Experimental Setup
3.2. Microbial Burden across Cardinal Directions
3.3. Materials Resistant to Fungal Growth
3.4. Dominant Species
3.5. Materials Characterisation
4. Conclusions
- On most of the investigated materials where a dominant morphology was present, Aureobasidium sp. was the dominant genus, implying that Aureobasidium can establish dominance already during the early phases of colonisation.
- Both the material type and the climate condition at the exposure site influenced fungal colonisation. Samples exposed in another climate zone or at a different time of the year might exhibit different infestation patterns. Therefore, the current findings can only be interpreted within the experimental setting used.
- Coated materials were generally less susceptible to fungal infestation. The distributions of the CFU counts on samples exposed in four directions differed significantly. The least growth was observed in the south, while the highest fungal burden was observed in the east and west.
- Based on described results, Aureobasidium sp. is considered a candidate for a living component of a new nature-inspired coating system designed to effectively protect architectonic surfaces. We assume that due to its polyextremotolerance, Aureobasidium sp. would be a good candidate for living coatings to be used in different climate zones. Nonetheless, further research in diverse climatic regions might identify alternative species and/or even polymicrobial communities as candidates for living coatings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, O. Wood and Tree Fungi: Biology, Damage, Protection, and Use, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Hill, C.; Kymäläinen, M.; Rautkari, L. Review of the Use of Solid Wood as an External Cladding Material in the Built Environment. J. Mater. Sci. 2022, 57, 9031–9076. [Google Scholar] [CrossRef]
- Kougoulis, J.S.; Kaps, R.; Walsh, B.; Bojczuk, K.; Crichton, T. Revision of EU European Ecolabel and Development of EU Green Public Procurement Criteria for Indoor and Outdoor Paints and Varnishes; EC Joint Research Center: Brussels, Belgium, 2012. [Google Scholar]
- Gibbons, M.J.; Nikafshar, S.; Saravi, T.; Ohno, K.; Chandra, S.; Nejad, M. Analysis of a Wide Range of Commercial Exterior Wood Coatings. Coatings 2020, 10, 1013. [Google Scholar] [CrossRef]
- Löfflath, F.; Gebhard, M. Rheological Changes during the Drying of a Waterborne Latex Coating. J. Coat. Technol. 1997, 69, 55–66. [Google Scholar] [CrossRef]
- Van Der Kooij, H.M.; Fokkink, R.; Van Der Gucht, J.; Sprakel, J. Quantitative Imaging of Heterogeneous Dynamics in Drying and Aging Paints. Sci. Rep. 2016, 6, srep34383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samyn, P.; Bosmans, J.; Cosemans, P. Current Alternatives for In-Can Preservation of Aqueous Paints: A Review. Mater. Proc. 2021, 7, 18. [Google Scholar]
- Schultz, T.P.; Nicholas, D.D.; Preston, A.F. A Brief Review of the Past, Present and Future of Wood Preservation. Pest Manag. Sci. 2007, 63, 784–788. [Google Scholar] [CrossRef]
- Broda, M. Natural Compounds for Wood Protection against Fungi—A Review. Molecules 2020, 25, 3538. [Google Scholar] [CrossRef]
- Guillitte, O. Bioreceptivity: A New Concept for Building Ecology Studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Sanmartín, P.; Miller, A.Z.; Prieto, B.; Viles, H.A. Revisiting and Reanalysing the Concept of Bioreceptivity 25 Years On. Sci. Total Environ. 2021, 770, 145314. [Google Scholar] [CrossRef]
- Sailer, M.F.; van Nieuwenhuijzen, E.J.; Knol, W. Forming of a Functional Biofilm on Wood Surfaces. Ecol. Eng. 2010, 36, 163–167. [Google Scholar] [CrossRef]
- Sandak, A. Engineered Living Materials for Sustainable and Resilient Architecture. Nat. Rev. Mater. 2023, 8, 357–359. [Google Scholar] [CrossRef]
- Podgorski, L.; Reynaud, C.; Montibus, M. Fungal Growth on Coated Wood Exposed Outdoors: Influence of Coating Pigmentation, Cardinal Direction, and Inclination of Wood Surfaces. Coatings 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Poohphajai, F.; Sandak, J.; Sailer, M.; Rautkari, L.; Belt, T.; Sandak, A. Bioinspired Living Coating System in Service: Evaluation of the Wood Protected with Biofinish during One-Year Natural Weathering. Coatings 2021, 11, 701. [Google Scholar] [CrossRef]
- Pfeffer, A.; Hoegger, P.J.; Kües, U.; Militz, H. Fungal Colonisation of Outside Weathered Modified Wood. Wood Sci. Technol. 2012, 46, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Gobakken, L.R.; Westin, M. Surface Mould Growth on Five Modified Wood Substrates Coated with Three Different Coating Systems When Exposed Outdoors. Int. Biodeterior. Biodegrad. 2008, 62, 397–402. [Google Scholar] [CrossRef]
- Sjökvist, T.; Blom, Å. The Influence of Coating Color, Heartwood and Sapwood, on Moisture Content and Growth of Microorganisms on the Surface during Outdoor Exposure of Norway Spruce Boards. J. Coat. Technol. Res. 2019, 16, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Sandak, A.; Sandak, J.; Noël, M.; Dimitriou, A. A Method for Accelerated Natural Weathering of Wood Subsurface and Its Multilevel Characterization. Coatings 2021, 11, 126. [Google Scholar] [CrossRef]
- Niklewski, J.; Sandak, J.; Bester Van Niekerk, P.; Brischke, C.; Acquah, R.; Sandak, A. Simplified Environmental Analysis of the Long-Term Performance of Wood Cladding and Decking. In Proceedings of the World Conference on Timber Engineering (WCTE 2023), Oslo, Norway, 19 June 2023; pp. 548–557. [Google Scholar]
- Sandak, A.; Sandak, J.; Brzezicki, M.; Kutnar, A. Bio-Based Building Skin; Springer Nature: Singapore, 2019. [Google Scholar]
- Riddell, R.W. Permanent Stained Mycological Preparations Obtained by Slide Culture. Mycologia 1950, 42, 265–270. [Google Scholar] [CrossRef]
- Gerrits Van Den Ende, A.H.G.G.; De Hoog, G.S. Variability and Molecular Diagnostics of the Neurotropic Species Cladophialophora Bantiana. Stud. Mycol. 1999, 1999, 151–162. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A Method for Designing Primer Sets for Speciation Studies in Filamentous Ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Hubka, V.; Kolarik, M. β-Tubulin Paralogue TubC Is Frequently Misidentified as the BenA Gene in Aspergillus Section Nigri Taxonomy: Primer Specificity Testing and Taxonomic Consequences. Persoonia Mol. Phylogeny Evol. Fungi 2012, 29, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Glass, N.L.; Donaldson, G.C. Development of Primer Sets Designed for Use with the PCR to Amplify Conserved Genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Liauw, C.M.; Slate, A.J.; Butler, J.A.; Wilson-Nieuwenhuis, J.S.T.; Deisenroth, T.; Preuss, A.; Verran, J.; Whitehead, K.A. The Effect of Surface Hydrophobicity on the Attachment of Fungal Conidia to Substrates of Polyvinyl Acetate and Polyvinyl Alcohol. J. Polym. Environ. 2020, 28, 1450–1464. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, K.A.; Liauw, C.M.; Lynch, S.; El Mohtadi, M.; Amin, M.; Preuss, A.; Deisenroth, T.; Verran, J. Diverse Surface Properties Reveal That Substratum Roughness Affects Fungal Spore Binding. iScience 2021, 24, 102333. [Google Scholar] [CrossRef]
- Poohphajai, F.; Myronycheva, O.; Karlsson, O.; Belt, T.; Rautkari, L.; Sandak, J.; Gubenšek, A.; Zalar, P.; Gunde-Cimerman, N.; Sandak, A. Fungal Colonisation on Wood Surfaces Weathered at Diverse Climatic Conditions. Heliyon 2023, 9, e17355. [Google Scholar] [CrossRef] [PubMed]
- Sandak, J.; Sandak, A.; Riggio, M. Characterization and Monitoring of Surface Weathering on Exposed Timber Structures with a Multi-Sensor Approach. Int. J. Archit. Herit. 2015, 9, 674–688. [Google Scholar] [CrossRef]
- Kržišnik, D.; Lesar, B.; Thaler, N.; Humar, M. Influence of Natural and Artificial Weathering on the Colour Change of Different Wood and Wood-Based Materials. Forests 2018, 9, 488. [Google Scholar] [CrossRef] [Green Version]
- Rüther, P.; Jelle, B.P. Color Changes of Wood and Wood-Based Materials Due to Natural and Artificial Weathering. Wood Mater. Sci. Eng. 2013, 8, 13–25. [Google Scholar] [CrossRef]
- Zhou, X.; Kubilay, A.; Derome, D.; Carmeliet, J. Comparison of Wind-Driven Rain Load on Building Facades in the Urban Environment and Open Field: A Case Study on Two Buildings in Zurich, Switzerland. Build. Environ. 2023, 233, 110038. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009; Volume 519. [Google Scholar]
- Gobakken, L.R.; Bardage, S.L.; Long, C.J., II. Succession of Staining Fungi on Acetylated Wood and the Effect of Selected Influencing Factors. In Proceedings of the 7th Meeting of the Nordic-Baltic Network in Wood Material Science & Engineering (WSE), Oslo, Norway, 27–28 October 2011; pp. 13–18. [Google Scholar]
- Robinson, J.R.; Isikhuemhen, O.S.; Anike, F.N. Fungal–Metal Interactions: A Review of Toxicity and Homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef]
- Fleurat-Lessard, P.; Dédaldéchamp, F.; Thibault, F.; Béré, E.; Roblin, G. Antifungal Effects of Iron Sulfate on Grapevine Fungal Pathogens. Sci. Hortic. 2011, 130, 517–523. [Google Scholar] [CrossRef]
- Sèbe, G.; Brook, M.A. Hydrophobization of Wood Surfaces: Covalent Grafting of Silicone Polymers. Wood Sci. Technol. 2001, 35, 269–282. [Google Scholar] [CrossRef]
- Mai, C.; Militz, H. Modification of Wood with Silicon Compounds. Treatment Systems Based on Organic Silicon Compounds-A Review. Wood Sci. Technol. 2004, 37, 453–461. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing Fungal Growth in Wood by Titanium Dioxide Nanoparticles. Int. Biodeterior. Biodegrad. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- Mazela, B. Changes of Copper and Chromium Content in Wood Impregnated with the Ccb and Cb Preservatives after Leaching. Folia For. 2000, B, 53–68. [Google Scholar]
- Quaranta, D.; Krans, T.; Santo, C.E.; Elowsky, C.G.; Domaille, D.W.; Chang, C.J.; Grass, G. Mechanisms of Contact-Mediated Killing of Yeast Cells on Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 416–426. [Google Scholar] [CrossRef] [Green Version]
- U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs. Reregistration Eligibility Decision (RED) for Coppers; U.S. Environmental Protection Agency: Washington, DC, USA, 2009.
- Baudy, P.; Konschak, M.; Sakpal, H.; Baschien, C.; Schulz, R.; Bundschuh, M.; Zubrod, J.P. The Fungicide Tebuconazole Confounds Concentrations of Molecular Biomarkers Estimating Fungal Biomass. Bull. Environ. Contam. Toxicol. 2020, 105, 620–625. [Google Scholar] [CrossRef]
- Peyton, L.R.; Gallagher, S.; Hashemzadeh, M. Triazole Antifungals: A Review. Drugs Today 2015, 51, 705–718. [Google Scholar] [CrossRef]
- Adamopoulos, F.G.; Vouvoudi, E.C.; Achilias, D.S.; Karapanagiotis, I. Fluorosilane Water-Repellent Coating for the Protection of Marble, Wood and Other Materials. Heritage 2021, 4, 2668–2675. [Google Scholar] [CrossRef]
- Schmidt, O.; Wei, D.S.; Tang, T.K.H.; Liese, W. Bamboo and Fungi. J. Bamboo Ratt. 2013, 12, 1–14. [Google Scholar]
- Gostinčar, C.; Grube, M.; De Hoog, S.; Zalar, P.; Gunde-Cimerman, N. Extremotolerance in Fungi: Evolution on the Edge. FEMS Microbiol. Ecol. 2010, 71, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Gostinčar, C.; Ohm, R.A.; Kogej, T.; Sonjak, S.; Turk, M.; Zajc, J.; Zalar, P.; Grube, M.; Sun, H.; Han, J.; et al. Genome Sequencing of Four Aureobasidium Pullulans Varieties: Biotechnological Potential, Stress Tolerance, and Description of New Species. BMC Genom. 2014, 15, 549. [Google Scholar] [CrossRef] [Green Version]
- van Nieuwenhuijzen, E.J.; Houbraken, J.A.M.P.; Meijer, M.; Adan, O.C.G.; Samson, R.A. Aureobasidium Melanogenum: A Native of Dark Biofinishes on Oil Treated Wood. Antonie Leeuwenhoek 2016, 109, 661–683. [Google Scholar] [CrossRef] [Green Version]
- Zalar, P.; Gostinčar, C.; de Hoog, G.S.; Uršič, V.; Sudhadham, M.; Gunde-Cimerman, N. Redefinition of Aureobasidium Pullulans and Its Varieties. Stud. Mycol. 2008, 61, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Yates, M.V.; Nakatsu, C.H.; Miller, R.V.; Pillai, S.D. Manual of Environmental Microbiology; ASM Press: New York, NY, USA, 2016. [Google Scholar]
- Patel, T.Y.; Buttner, M.; Rivas, D.; Cross, C.; Bazylinski, D.A.; Seggev, J. Variation in Airborne Fungal Spore Concentrations among Five Monitoring Locations in a Desert Urban Environment. Environ. Monit. Assess. 2018, 190, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skjøth, C.A.; Damialis, A.; Belmonte, J.; De Linares, C.; Fernández-Rodríguez, S.; Grinn-Gofroń, A.; Jędryczka, M.; Kasprzyk, I.; Magyar, D.; Myszkowska, D.; et al. Alternaria Spores in the Air across Europe: Abundance, Seasonality and Relationships with Climate, Meteorology and Local Environment. Aerobiologia 2016, 32, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The Genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandak, A.; Földvári-Nagy, E.; Poohphajai, F.; Diaz, R.H.; Gordobil, O.; Sajinčič, N.; Ponnuchamy, V.; Sandak, J. Hybrid Approach for Wood Modification: Characterization and Evaluation of Weathering Resistance of Coatings on Acetylated Wood. Coatings 2021, 11, 658. [Google Scholar] [CrossRef]
- Gobakken, L.R.; Vestøl, G.I. Surface Mould and Blue Stain Fungi on Coated Norway Spruce Cladding. Int. Biodeterior. Biodegrad. 2012, 75, 181–186. [Google Scholar] [CrossRef]
- Žlahtič, M.; Humar, M. Influence of Artificial and Natural Weathering on the Hydrophobicity and Surface Properties of Wood. Bioresources 2016, 11, 4964–4989. [Google Scholar] [CrossRef] [Green Version]
- Veeger, M.; Ottelé, M.; Prieto, A. Making Bioreceptive Concrete: Formulation and Testing of Bioreceptive Concrete Mixtures. J. Build. Eng. 2021, 44, 102545. [Google Scholar] [CrossRef]
- Butina Ogorelec, K.; Gubenšek, A.; Poohphajai, F.; Sandak, A. Assessing the bioreceptivity of biobased cladding materials. Zenodo 2023, 9, 8137772. [Google Scholar] [CrossRef]




| Sample nr. | Group 1 | Sample Information | Coating | Species |
|---|---|---|---|---|
| 1 | therm | Treated in 212 °C | no | spruce |
| 2 | hybr | Treated in 212 °C with addition of FeSO4 | no | spruce |
| 3 | natr | Heartwood kiln dried 50–70 °C | no | pine |
| 4 | surf | Oiled stick-glued bamboo façade profiles | no | bamboo |
| 5 | impr | Wood impregnated with silicone and silicate | no | beech |
| 6 | hybr | Thermally treated and coated (industrially) Semi-transparent coating | yes | spruce |
| 7 | chem | Furfurylated (industrially) | no | pine |
| 8 | impr | Impregnated with TO2 nanoparticles | no | pine |
| 9 | hybr | Impregnated with TO2 nanoparticles and linseed oil | no | pine |
| 10 | hybr | Thermally treated and impregnated with copper oxide, boric acid, and tebuconazole | no | pine |
| 11 | natr | Air dried | no | beech |
| 12 | therm | Hydro-thermally treated (industrially) | no | frake |
| 13 | chem | Acetylated (industrially) | no | beech |
| 14 | hybr | Thermally treated and coated (industrially) Semi-transparent coating | yes | frake |
| 15 | impr | Soaked with concentrated fluorosilane | no | poplar |
| 16 | impr | Melamine treated | no | poplar |
| 17 | comp | Coated acetylated MDF (industrially) Opaque petroleum grey coating | yes | composite |
| 18 | surf | Coated with solvent-based product (industrially) Semi-transparent coating | yes | pine |
| 19 | impr | Treated with copper ethanolamine | no | spruce |
| 20 | comp | Coated acetylated MDF (industrially) Opaque white coating | yes | composite |
| 21 | hybr | Acetylated and coated (industrially) Opaque white coating | yes | pine |
| 22 | hybr | Acetylated and coated (industrially) Semi-transparent coating | yes | pine |
| 23 | natr | Natural fir (kiln dried) | no | fir |
| 24 | therm | Thermally treated fir (vacuum) | no | fir |
| 25 | surf | Coated (industrially)Transparent coating | yes | fir |
| 26 | hybr | Coated thermally treated fir (industrially) Transparent coating | yes | fir |
| 27 | surf | Coated natural oak (industrially) Transparent coating | yes | oak |
| 28 | hybr | Coated thermally treated oak (industrially) Transparent coating | yes | oak |
| 29 | natr | Natural oak (kiln dried) | no | oak |
| 30 | therm | Thermally treated oak (vacuum) | no | oak |
| 31 | surf | Nanoparticle transparent coating, 3 layers (DIY application) | yes | pine |
| 32 | comp | Wood–plastic composite | no | composite |
| 33 | therm | Over-thermally treated spruce | no | spruce |
| Sample | Antifungal Treatment/Property | Mode of Action |
|---|---|---|
| 2, thermally treated and impregnated | Iron(II) sulphate | Excess iron leads to disturbed iron homeostasis [40,41] |
| 5, impregnated | Silicone and silicate | Acts as a water repellent and reduces moisture uptake of wood [42,43]. |
| 8, impregnated 9, impregnated | Titanium dioxide nanoparticles | Generation of free radicals (hydroxyl and superoxide anion) and hydrogen peroxide causing microbial growth reductions [44] |
| 10, thermally treated and impregnated | Copper oxide, boric acid, and tebuconazole [45] | Copper can cause membrane damage [46] and protein denaturation [47]. Tebuconazole leads to ergosterol degradation [48,49]. |
| 15, impregnated | Fluorosilane | Acts as a water repellent and reduces moisture uptake of wood [50]. |
| 19, impregnated | Copper ethanolamine | Copper can cause membrane damage [46] and protein denaturation [47]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butina Ogorelec, K.; Gubenšek, A.; Poohphajai, F.; Sandak, A. Assessing the Bioreceptivity of Biobased Cladding Materials. Coatings 2023, 13, 1413. https://doi.org/10.3390/coatings13081413
Butina Ogorelec K, Gubenšek A, Poohphajai F, Sandak A. Assessing the Bioreceptivity of Biobased Cladding Materials. Coatings. 2023; 13(8):1413. https://doi.org/10.3390/coatings13081413
Chicago/Turabian StyleButina Ogorelec, Karen, Ana Gubenšek, Faksawat Poohphajai, and Anna Sandak. 2023. "Assessing the Bioreceptivity of Biobased Cladding Materials" Coatings 13, no. 8: 1413. https://doi.org/10.3390/coatings13081413
APA StyleButina Ogorelec, K., Gubenšek, A., Poohphajai, F., & Sandak, A. (2023). Assessing the Bioreceptivity of Biobased Cladding Materials. Coatings, 13(8), 1413. https://doi.org/10.3390/coatings13081413