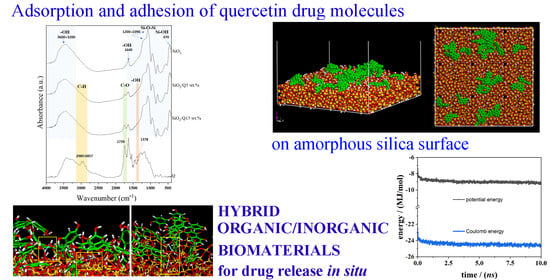
Hybrid Organic–Inorganic Biomaterials as Drug Delivery Systems: A Molecular Dynamics Study of Quercetin Adsorption on Amorphous Silica Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. SiO2 and SiO2/Quercetin (5 and 15 wt.%) Sol–Gel Synthesis
2.2.2. Fourier Transform Infrared Analysis
2.2.3. Molecular Mechanics and Dynamics Methods
3. Results and Discussion
3.1. Adsorption of a Single Quercetin Molecule on the Amorphous SiO2 Surface
3.2. Quercetin Molecules on the Amorphous SiO2 Surface: Small Drug Concentration
3.3. Quercetin Molecules on the Amorphous SiO2 Surface: Higher Drug Concentration
3.4. FT–IR Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSN | Mesoporous silica nanoparticle |
| MM | Molecular Mechanics |
| MD | Molecular Dynamics |
| NVT ensemble | Number of particles, Volume, and Temperature is constant |
| c.o.m. | center of mass |
References
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: Antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 2011, 9, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Gao, M.; Yun, Y.; Malmsten, M.; Rotello, V.M.; Zboril, R.; Akhavan, O.; Kraskouski, A.; Amalraj, J.; Cai, X.; et al. Antibacterial Nanomaterials: Mechanisms, Impacts on Antimicrobial Resistance and Design Principles. Angew. Chem. Int. Ed. 2023, 62, e202217345. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Perez, D.; Guarch-Pérez, C.; Purbayanto, M.A.K.; Choinska, E.; Riool, M.; Zaat, S.A.J.; Swieszkowski, W. 3D-printed dual drug delivery nanoparticle-loaded hydrogels to combat antibiotic-resistant bacteria. Int. J. Bioprint. 2023, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updates 2023, 68, 100954. [Google Scholar] [CrossRef] [PubMed]
- Brasier, N.; Ates, H.C.; Sempionatto, J.R.; Cotta, M.O.; Widmer, A.F.; Eckstein, J.; Goldhahn, J.; Roberts, J.A.; Gao, W.; Dincer, C. A three-level model for therapeutic drug monitoring of antimicrobials at the site of infection. Lancet Infect. Dis. 2023, 23, E445–E453. [Google Scholar] [CrossRef]
- Schwach-Abdellaoui, K.; Vivien-Castioni, N.; Gurny, R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur. J. Pharm. Biopharm. 2000, 50, 83–99. [Google Scholar] [CrossRef]
- Myhrman, E.; Håkansson, J.; Lindgren, K.; Björn, C.; Sjöstrand, V.; Mahlapuu, M. The novel antimicrobial peptide PXL150 in the local treatment of skin and soft tissue infections. Appl. Microbiol. Biotechnol. 2013, 97, 3085–3096. [Google Scholar] [CrossRef]
- Huang, J.; Sebastian, S.; Collin, M.; Tägil, M.; Lidgren, L.; Raina, D.B. A calcium sulphate/hydroxyapatite ceramic biomaterial carrier for local delivery of tobramycin in bone infections: Analysis of rheology, drug release and antimicrobial efficacy. Ceram. Int. 2023, 49, 33725–33734. [Google Scholar] [CrossRef]
- Caplin, J.D.; García, A.J. Implantable antimicrobial biomaterials for local drug delivery in bone infection models. Acta Biomater. 2019, 93, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Mulazzi, M.; Campodoni, E.; Bassi, G.; Montesi, M.; Panseri, S.; Bonvicini, F.; Gentilomi, G.A.; Tampieri, A.; Sandri, M. Medicated Hydroxyapatite/Collagen Hybrid Scaffolds for Bone Regeneration and Local Antimicrobial Therapy to Prevent Bone Infections. Pharmaceutics 2021, 13, 1090. [Google Scholar] [CrossRef] [PubMed]
- Young, B.C.; Dudareva, M.; Vicentine, M.P.; Hotchen, A.J.; Ferguson, J.; McNally, M. Microbial Persistence, Replacement and Local Antimicrobial Therapy in Recurrent Bone and Joint Infection. Antibiotics 2023, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Tandberg, F.; Liu, Y.; Raina, D.B.; Tägil, M.; Collin, M.; Lidgren, L. Extended local release and improved bacterial eradication by adding rifampicin to a biphasic ceramic carrier containing gentamicin or vancomycin. Bone Jt. Res. 2022, 11, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Nishio Ayre, W.; Birchall, J.C.; Evans, S.L.; Denyer, S.P. A novel liposomal drug delivery system for PMMA bone cements. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1510–1524. [Google Scholar] [CrossRef]
- Levack, A.E.; Turajane, K.; Yang, X.; Miller, A.O.; Carli, A.V.; Bostrom, M.P.; Wellman, D.S. Thermal Stability and in Vitro Elution Kinetics of Alternative Antibiotics in Polymethylmethacrylate (PMMA) Bone Cement. J. Bone Jt. Surg. 2021, 103, 1694–1704. [Google Scholar] [CrossRef]
- Lukaviciute, L.; Karciauskaite, J.; Grigoraviciute, I.; Vasiliauskiene, D.; Sokol, D.; Kareiva, A. Calcium Hydroxyapatite Coatings: Low-Temperature Synthesis and Investigation of Antibacterial Properties. Coatings 2023, 13, 1991. [Google Scholar] [CrossRef]
- Longo, R.; Raimondo, M.; Vertuccio, L.; Ciardulli, M.C.; Sirignano, M.; Mariconda, A.; Della Porta, G.; Guadagno, L. Bottom-Up Strategy to Forecast the Drug Location and Release Kinetics in Antitumoral Electrospun Drug Delivery Systems. Int. J. Mol. Sci. 2023, 24, 1507. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vertuccio, L.; Lamparelli, E.P.; Ciardulli, M.C.; Longo, P.; Mariconda, A.; Della Porta, G.; Longo, R. Electrospun Membranes Designed for Burst Release of New Gold-Complexes Inducing Apoptosis of Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 7147. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Yang, K.; Chen, Z.; Li, Q.; Zheng, L.; Wu, Z.; Zhang, X.; Su, L.; Wu, Y.; et al. Drug-Free Antimicrobial Nanomotor for Precise Treatment of Multidrug-Resistant Bacterial Infections. Nano Lett. 2023, 23, 3929–3938. [Google Scholar] [CrossRef]
- Talat, A.; Khan, A.U. Artificial intelligence as a smart approach to develop antimicrobial drug molecules: A paradigm to combat drug-resistant infections. Drug Discov. Today 2023, 28, 103491. [Google Scholar] [CrossRef]
- Qin, X.; Zheng, J.; Yang, X.; Gong, W.; Luo, L.P.; Ji, L. Bioactivity of a gelatin/organic-inorganic hybrid biomaterial fibre enhanced by metronidazole release. Mater. Lett. 2022, 325, 132803. [Google Scholar] [CrossRef]
- Doraiswamy, A.; Jin, C.; Narayan, R.J.; Mageswaran, P.; Mente, P.; Modi, R.; Auyeung, R.; Chrisey, D.B.; Ovsianikov, A.; Chichkov, B. Two photon induced polymerization of organic-inorganic hybrid biomaterials for microstructured medical devices. Acta Biomater. 2006, 2, 267–275. [Google Scholar] [CrossRef]
- Andrade, G.; Barbosa-Stancioli, E.F.; Mansur, A.A.P.; Vasconcelos, W.L.; Mansur, H.S. Design of novel hybrid organic-inorganic nanostructured biomaterials for immunoassay applications. Biomed. Mater. 2006, 1, 221–234. [Google Scholar] [CrossRef]
- Duan, L.; Li, X.; Ji, R.; Hao, Z.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Nanoparticle-Based Drug Delivery Systems: An Inspiring Therapeutic Strategy for Neurodegenerative Diseases. Polymers 2023, 15, 2196. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Li, J.; Jiang, Y.; Xu, K.; Su, J. Bone-Targeted Nanoparticle Drug Delivery System: An Emerging Strategy for Bone-Related Disease. Front. Pharmacol. 2022, 13, 909408. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.-G.; Sun, T. Nanoparticle-Based Drug Delivery Systems for Induction of Tolerance and Treatment of Autoimmune Diseases. Front. Bioeng. Biotechnol. 2022, 19, 889291. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood-brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, K.; Hoffmann, M.M.; Dreis, S.; Herbert, E.; Alyautdin, R.N.; Michaelis, M.; Kreuter, J.; Langer, K. Covalent linkage of apolipoprotein E to albumin nanoparticles strongly enhances drug transport into the brain. J. Pharmacol. Exp. Ther. 2006, 317, 1246–1253. [Google Scholar] [CrossRef]
- Kreuter, J.; Hekmatara, T.; Dreis, S.; Vogel, T.; Gelperina, S.; Langer, K. Covalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J. Control. Release 2007, 118, 54–58. [Google Scholar] [CrossRef]
- Peetla, C.; Vijayaraghavalu, S.; Labhasetwar, V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 1686–1698. [Google Scholar] [CrossRef]
- Partlow, K.C.; Lanza, G.M.; Wickline, S.A. Exploiting lipid raft transport with membrane targeted nanoparticles: A strategy for cytosolic drug delivery. Biomaterials 2008, 29, 3367–3375. [Google Scholar] [CrossRef]
- Ossola, B.; Kääriäinen, T.M.; Raasmaja, A.; Männistö, P.T. Time-dependent protective and harmful effects of quercetin on 6-OHDA-induced toxicity in neuronal SH-SY5Y cells. Toxicology 2008, 250, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chena, R.; Lin, J.; Hong, J.; Han, D.; Zhang, A.D.; Lan, R.; Fu, L.; Wu, Z.; Lin, J.; Zhang, W.; et al. Potential toxicity of quercetin: The repression of mitochondrial copy number via decreased POLG expression and excessive TFAM expression in irradiated murine bone marrow. Toxicol. Rep. 2014, 1, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Baldrighi, M.; Trusel, M.; Tonini, R.; Giordani, S. Carbon Nanomaterials Interfacing with Neurons: An In vivo Perspective. Front. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Mohammadinejad, R.; Kailasa, S.K.; Ahmadi, Z.; Afshar, E.G.; Pardakhty, A. Carbon dots as versatile nanoarchitectures for the treatment of neurological disorders and their theranostic applications: A review. Adv. Colloid Interface Sci. 2020, 278, 102123. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Knowles, J.C.; Kim, H.-W. Advances in nanoparticle development for improved therapeutics delivery: Nanoscale topographical aspect. J. Tissue Eng. 2019, 10, 2041731419877528. [Google Scholar] [CrossRef]
- Khan, S.; Dunphy, A.; Anike, M.S.; Belperain, S.; Patel, K.; Chiu, N.H.L.; Jia, Z. Recent Advances in Carbon Nanodots: A Promising Nanomaterial for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 6786. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941. [Google Scholar] [CrossRef]
- Lee, H.P.; Gaharwar, A.K. Light-Responsive Inorganic Biomaterials for Biomedical Applications. Adv. Sci. 2020, 7, 2000863. [Google Scholar] [CrossRef]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A Review of Organic and Inorganic Biomaterials for Neural Interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, Z.H.; Che, S. Mesostructured chitosan-silica hybrid as a biodegradable carrier for a pH-responsive drug delivery system. Dalton Trans. 2012, 41, 5038–5044. [Google Scholar] [CrossRef]
- Peveler, W.J.; Bear, J.C.; Southern, P.; Parkin, I.P. Organic-inorganic hybrid materials: Nanoparticle containing organogels with myriad applications. ChemComm 2014, 50, 14418–14420. [Google Scholar] [CrossRef]
- Wang, T.; Leng, B.; Che, R.C.; Shao, Z. Biomimetic Synthesis of Multilayered Aragonite Aggregates Using Alginate as Crystal Growth Modifier. Langmuir 2010, 26, 13385–13392. [Google Scholar] [CrossRef]
- Zhou, L.; O’Brien, P. Mesocrystals: A New Class of Solid Materials. Small 2008, 4, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Nagai, M.; Seo, W.S.; Koumoto, K. Template-free self-assembly of a nanoporous TiO2 thin film. J. Am. Ceram. Soc. 2007, 90, 831–837. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering mesoporous silica nanoparticles for drug delivery: Where are we after two decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Shi, R. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Transduct. Target Ther. 2023, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.E.; Sadeghi, M.; Mansouri-Torshizi, H.; Saidifar, M. High cancer selectivity and improving drug release from mesoporous silica nanoparticles in the presence of human serum albumin in cisplatin, carboplatin, oxaliplatin, and oxalipalladium treatment. Eur. J. Pharm. Sci. 2023, 187, 106477. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhai, J.; Xiao, Q.; Zhao, S.; Li, C. Polysaccharide/mesoporous silica nanoparticle-based drug delivery systems: A review. Int. J. Biol. Macromol. 2021, 193, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.H.; Smatt, J.-H.; Govardhanam, N.P.; Ibrahim, H.M.; Ismael, H.R.; Afouna, M.I.; Samy, A.M.; Rosenholm, J.M. Formulation and optimization of drug-loaded mesoporous silica nanoparticle-based tablets to improve the dissolution rate of the poorly water-soluble drug silymarin. Eur. J. Pharm. Sci. 2020, 142, 105103. [Google Scholar] [CrossRef]
- He, H.; Xiao, H.; Kuang, H.; Xie, Z.; Chen, X.; Jing, X.; Huang, Y. Synthesis of mesoporous silica nanoparticle-oxaliplatin conjugates for improved anticancer drug delivery. Colloids Surf. B 2014, 117, 75–81. [Google Scholar] [CrossRef]
- Miao, Y.; Feng, Y.; Bai, J.; Liu, Z.; Zhao, X.J. Optimized mesoporous silica nanoparticle-based drug delivery system with removable manganese oxide gatekeeper for controlled delivery of doxorubicin. Colloid Interface Sci. 2021, 592, 227–236. [Google Scholar] [CrossRef]
- Ahmed, H.; Gomte, S.S.; Prathyusha, E.; Prabakaran, A.; Agrawal, M.; Alexander, A. Biomedical applications of mesoporous silica nanoparticles as a drug delivery carrier. J. Drug Deliv. Sci. Technol. 2022, 76, 103729. [Google Scholar] [CrossRef]
- Tourne-Peteilh, C.; Begu, S.; Lerner, D.A.; Galarneau, A.; Lafont, U.; Devoisselle, J.-M. Sol-gel one-pot synthesis in soft conditions of mesoporous silica materials ready for drug delivery system. J. Solgel Sci. Technol. 2012, 61, 455–462. [Google Scholar] [CrossRef]
- Alhendal, A.; Rashad, M.; Husain, A.; Seyyal, E.; Mouffouk, F.; Makhseed, S. Efficient sol-gel immobilization of microporous polymer on silica-based adsorbent for the enrichment of non-steroidal anti-inflammatory drugs. Microporous Mesoporous Mater. 2022, 344, 112152. [Google Scholar] [CrossRef]
- Rani, R.; Malik, P.; Dhania, S.; Mukherjee, T.K. Recent Advances in Mesoporous Silica Nanoparticle-Mediated Drug Delivery for Breast Cancer Treatment. Pharmaceutics 2023, 15, 227. [Google Scholar] [CrossRef]
- Niu, N.; He, F.; Ma, P.; Gai, S.; Yang, G.; Qu, F.; Wang, Y.; Xu, J.; Yang, P. Up-Conversion Nanoparticle Assembled Mesoporous Silica Composites: Synthesis, Plasmon-Enhanced Luminescence, and Near-Infrared Light Triggered Drug Release. ACS Appl. Mater. Interfaces 2014, 6, 3250–3262. [Google Scholar] [CrossRef]
- Stephen, S.; Gorain, B.; Choudhury, H.; Chatterjee, B. Exploring the role of mesoporous silica nanoparticle in the development of novel drug delivery systems. Drug Deliv. Transl. Res. 2022, 12, 105–123. [Google Scholar] [CrossRef]
- Feng, Y.; Li, N.-X.; Yin, H.-L.; Chen, T.-Y.; Yang, Q.; Wu, M. Thermo-and pH-responsive, Lipid-coated, Mesoporous Silica Nanoparticle-based Dual Drug Delivery System to Improve the Antitumor Effect of Hydrophobic Drugs. Mol. Pharm. 2019, 16, 422–436. [Google Scholar] [CrossRef]
- Li, X.; He, Q.; Shi, J. Global Gene Expression Analysis of Cellular Death Mechanisms Induced by Mesoporous Silica Nanoparticle-Based Drug Delivery System. ACS Nano 2014, 8, 1309–1320. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.Z.; Tang, Q.D.M.; Hu, J.; Yang, D. Reduction-responsive drug delivery based on mesoporous silica nanoparticle core with crosslinked poly(acrylic acid) shell. Mater. Sci. Eng. C 2013, 33, 3426–3431. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Elsevier Inc.: Amsterdam, The Netherlands, 1990; ISBN 978-0-08-057103-4. [Google Scholar] [CrossRef]
- Kozina, A.; Espinoza, K.A.; Ortiz-Islas, E.; Rivero, I.A. Effect of Thermal Treatment of Sol-Gel Titania Reservoirs on Release Kinetics of Phenytoin. J. Am. Ceram. Soc. 2012, 95, 2022–2027. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Correia, A.; Yang, Y.Y.; Zheng, K.; Liu, D.; Schubert, D.W.; Boccaccini, A.R.; Santos, H.A.; Roether, J.A. Electrospun Polyhydroxybutyrate/Poly(ε-caprolactone)/Sol-Gel-Derived Silica Hybrid Scaffolds with Drug Releasing Function for Bone Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 14540–14548. [Google Scholar] [CrossRef]
- Moral, A.; Borrull, F.; Furton, K.G.; Kabir, A.; Fontanals, N.; Marce, R.M. Development of sol-gel silica-based mixed-mode zwitterionic sorbents for determining drugs in environmental water samples. J. Chromatogr. A 2022, 1676, 463237. [Google Scholar] [CrossRef]
- Vueba, M.L.; Pina, M.E.; Veiga, F.; Sousa, J.J.; Batista de Carvalho, L.A.E. Conformational study of ketoprofen by combined DFT calculations and Raman spectroscopy. J. Pharm. 2006, 307, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Melisi, D.; Curcio, A.; Rimoli, M.G. Sol-gel processing of anti-inflammatory entrapment in silica, release kinetics, and bioactivity. J. Biomed. Mater. Res. A 2008, 87A, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R′′Si(OR′)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Capeletti, L.B.; Zimnoch, J.H. Fourier Transform Infrared and Raman Characterization of Silica-Based Materials. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences; Stauffer, M.T., Ed.; InTech: Greensburg, PA, USA, 2016; ISBN 978-953-51-2680-5. [Google Scholar]
- Almeida, R.M.; Pantano, C.G. Structural Investigation of Silica Gel Films by Infrared Spectroscopy. J. Appl. Phys. 1990, 68, 4225–4232. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric Identification of Organic Compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol-gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Deshmukh, K.; Kovárík, T.; Krenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent advances and future perspectives of sol-gel derived porous bioactive glasses: A review. RSC Adv. 2020, 10, 33782–33835. [Google Scholar] [CrossRef]
- Raffaini, G.; Catauro, M. Surface Interactions between Ketoprofen and Silica-Based Biomaterials as Drug Delivery System Synthesized via Sol-Gel: A Molecular Dynamics Study. Materials 2022, 15, 2759. [Google Scholar] [CrossRef]
- Raffaini, G.; Elli, S.; Ganazzoli, F. Computer simulation of bulk mechanical properties and surface hydration of biomaterials. J. Biomed. Mater. Res. A 2006, 77A, 618–626. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Surface Hydration of Polymeric (Bio)Materials: A Molecular Dynamics Simulation Study. J. Biomed. Mater. Res. Part A 2010, 92, 1382–1391. [Google Scholar] [CrossRef]
- Mantero, S.; Piuri, D.; Montevecchi, F.M.; Vesentini, S.; Ganazzoli, F.; Raffaini, G. Albumin adsorption onto pyrolytic carbon: A molecular mechanics approach. J. Biomed. Mater. Res. 2002, 59, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Raffaini, G.; Ganazzoli, F. Adsorption of charged albumin subdomains on a graphite surface. J. Biomed. Mater. Res. A 2006, 76A, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Mücksch, C.; Urbassek, H.M. Molecular Dynamics Simulation of Free and Forced BSA Adsorption on a Hydrophobic Graphite Surface. Langmuir 2011, 27, 12938–12943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Y. Molecular simulation of adsorption and its implications to protein chromatography: A review. Biochem. Eng. J. 2010, 48, 408–415. [Google Scholar] [CrossRef]
- Barinov, N.A.; Prokhorov, V.V.; Dubrovin, E.V.; Klinov, D.V. AFM visualization at a single-molecule level of denaturated states of proteins on graphite. Colloids Surf. B-Biointerfaces 2016, 146, 777–784. [Google Scholar] [CrossRef] [PubMed]
- De Leo, F.; Magistrato, A.; Bonifazi, D. Interfacing proteins with graphitic nanomaterials: From spontaneous attraction to tailored assemblies. Chem. Soc. Rev. 2015, 44, 6916–6953. [Google Scholar] [CrossRef] [PubMed]
- Ozboyaci, M.; Kokh, D.B.; Corni, S.; Wade, R.C. Modeling and simulation of protein-surface interactions: Achievements and challenges. Q. Rev. Biophys. 2016, 49, e4. [Google Scholar] [CrossRef]
- Karajanagi, S.S.; Yang, H.C.; Asuri, P.; Sellitto, E.; Dordick, J.S.; Kane, R.S. Protein-assisted solubilization of single-walled carbon nanotubes. Langmuir 2006, 22, 1392–1395. [Google Scholar] [CrossRef]
- Yang, J.; Shojaei, S.; Shojaei, S. Removal of drug and dye from aqueous solutions by graphene oxide: Adsorption studies and chemometrics methods. npj Clean Water 2022, 5, 5. [Google Scholar] [CrossRef]
- Xu, P.; Cao, J.; Yin, C.; Wang, L.; Wu, L. Quantum chemical study on the adsorption of megazol drug on the pristine BC3 nanosheet. Supramol. Chem. 2021, 33, 63–69. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, M.A.; Carabineiro, S.A.C. Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/β-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J. Hazard. Mater. 2021, 409, 124840. [Google Scholar] [CrossRef]
- Kerdpol, K.; Nutho, B.; Krusong, K.; Poo-arporn, R.P.; Rungrotmongkol, T.; Hannongbua, S. Encapsulation of α-tocopherol in large-ring cyclodextrin containing 26 α-D-glucopyranose units: A molecular dynamics study. J. Mol. Liq. 2021, 339, 116802. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Shukla, S.; Jakowski, J.; Kadian, S.; Narayan, R.J. Computational approaches to delivery of anticancer drugs with multidimensional nanomaterials. Comput. Struct. Biotechnol. J. 2023, 21, 4149–4158. [Google Scholar] [CrossRef]
- Raffaini, G.; Elli, S.; Ganazzoli, F.; Catauro, M. Inclusion Complexes Between β-cyclodextrin and the Anticancer Drug 5-Fluorouracil for its Solubilization: A Molecular Dynamics Study at Different Stoichiometries. Macromol. Symp. 2022, 404, 2100305. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Understanding Surface Interaction and Inclusion Complexes between Piroxicam and Native or Crosslinked beta-Cyclodextrins: The Role of Drug Concentration. Molecules 2020, 25, 2848. [Google Scholar] [CrossRef]
- Treccani, S.; Alongi, J.; Manfredi, A.; Ferruti, P.; Cavalli, R.; Raffaini, G.; Ranucci, E. L-Arginine-Derived Polyamidoamine Oligomers Bearing at Both Ends β-Cyclodextrin Units as pH-Sensitive Curcumin Carriers. Polymers 2022, 14, 3193. [Google Scholar] [CrossRef] [PubMed]
- Zagami, R.; Barattucci, A.; Scolaro, L.M.; Viale, M.; Raffaini, G.; Bonaccorsi, P.M.; Mazzaglia, A. Curcumin/amphiphilic cyclodextrin nanoassemblies: Theoretical and spectroscopic studies to address their debut in anticancer therapy. J. Mol. Liq. 2023, 389, 122841. [Google Scholar] [CrossRef]
- Pivato, R.V.; Rossi, F.; Ferro, M.; Castiglione, F.; Trotta, F.; Mele, A. beta-Cyclodextrin Nanosponge Hydrogels as Drug Delivery Nanoarchitectonics for Multistep Drug Release Kinetics. ACS Appl. Polym. Mater. 2021, 3, 6562–6571. [Google Scholar] [CrossRef]
- Dignam, J.D.; Qu, X.G.; Ren, J.; Chaires, J.B. Daunomycin binding to detergent micelles: A model system for evaluating the hydrophobic contribution to drug-DNA interactions. J. Phys. Chem. B 2007, 111, 11576–11584. [Google Scholar] [CrossRef] [PubMed]
- Raffaini, G. Adsorption and Self-Aggregation of Chiral [5]-Aza[6]helicenes on DNA Architecture: A Molecular Dynamics Study. J. Phys. Chem. B 2023, 127, 8285–8295. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Blanco, I.; Clarke, R.J.; Fiorentino, M.; D’Angelo, A.; Raffaini, G. Sol–Gel Synthesis, Thermal Characterization, Surface Interactions, and Release Kinetics of Silica/Drug Hybrid System. Macromol. Symp. 2022, 404, 2100262. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/Quercetin Sol– Gel Hybrids as Antioxidant Dental Implant Materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Latteri, A.; Cicala, G.; D’Angelo, A.; Viola, V.; Arconati, V.; Catauro, M. Antibacterial and Chemical Characterization of Silica-Quercetin-PEG Hybrid Materials Synthesized by Sol–Gel Route. Molecules 2022, 27, 979. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, V.; Dell’Anna, M.M.; Mastrorilli, P.; Viola, V.; Catauro, M.; D’Angelo, A. Synthesis by Sol–Gel Route of Organic–Inorganic Hybrid Material: Chemical Characterization and In Vitro Release Study. Appl. Sci. 2023, 13, 8410. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Chakraborty, P.K.K.; Adhikari, J.; Saha, P. Variation of the properties of sol–gel synthesized bioactive glass 45S5 in organic and inorganic acid catalysts. Mater. Adv. 2021, 2, 413–425. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Sorathiya, V. Phase change material and MXene composited refractive index sensor for a wide range of sensing applications at visible and infrared wavelength spectrum. Optik 2023, 272, 170242. [Google Scholar] [CrossRef]
- Materials Studio BIOVIA. Accelrys Inc. InsightII 2000; Accelrys Inc.: San Diego, CA, USA, 2000; Available online: http://www.accelrys.com (accessed on 4 April 2021).
- Dauber-Osguthorpe, P.; Roberts, V.A.; Osguthorpe, D.J.; Wolff, J.; Genest, M.; Hagler, A.T. Structure and energetics of ligand binding to proteins: Escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins Struct. Funct. Genet. 1988, 4, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Nejad, M.A.; Mücksch, C.; Urbassek, H.M. Insulin adsorption on crystalline SiO2: Comparison between polar and nonpolar surfaces using accelerated molecular-dynamics simulations. Chem. Phys. Lett. 2017, 670, 77–83. [Google Scholar] [CrossRef]
- Nejad, M.A.; Urbassek, H.M. Diffusion of cisplatin molecules in silica nanopores: Molecular dynamics study of a targeted drug delivery system. J. Mol. Graph. Model. 2019, 86, 228–234. [Google Scholar] [CrossRef]
- Catauro, M.; D’Angelo, A.; Fiorentino, M.; Giullifa, G.; Risoluti, R.; Vecchio Ciprioti, S. Thermal behavior, morphology and antibacterial properties study of silica/quercetin nanocomposite materials prepared by sol–gel route. J Therm. Anal. Calorim. 2022, 147, 5337–5350. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Gallicchio, M.; Pacifico, S. Influence of the polymer amount on bioactivity and biocompatibility of SiO2/PEG hybrid materials synthesized by sol–gel technique. Mater. Sci. Eng. Part C 2015, 48, 548–555. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Piccolella, S.; Pacifico, S. Sol–gel synthesis and characterization of SiO2/PCL hybrid materials containing quercetin as new materials for antioxidant implants. Mater. Sci. Eng. Part C 2016, 58, 945–952. [Google Scholar] [CrossRef]
- Raffaini, G. Surface Chemistry, Crystal Structure, Size and Topography Role in the Albumin Adsorption Process on TiO2 Anatase Crystallographic Faces and Its 3D-Nanocrystal: A Molecular Dynamics Study. Coatings 2021, 11, 420. [Google Scholar] [CrossRef]
- Shen, J.-W.; Wu, T.; Wang, Q.; Pan, H.H. Molecular simulation of protein adsorption and desorption on hydroxyapatite surfaces. Biomaterials 2008, 29, 513–532. [Google Scholar] [CrossRef]
- Utesch, T.; Daminelli, G.; Mroginski, M.A. Molecular Dynamics Simulations of the Adsorption of Bone Morphogenetic Protein-2 on Surfaces with Medical Relevance. Langmuir 2011, 27, 13144–13153. [Google Scholar] [CrossRef]
- Chong, W.L.; Chupradit, K.; Chin, S.P.; Khoo, M.M.; Khor, S.M.; Tayapiwatana, C.; Nimmanpipug, P.; Thongkum, W.; Lee, V.S. Protein-Protein Interactions: Insight from Molecular Dynamics Simulations and Nanoparticle Tracking Analysis. Molecules 2021, 26, 5696. [Google Scholar] [CrossRef]
- Martin, J.; Frezza, E. A dynamical view of protein-protein complexes: Studies by molecular dynamics simulations. Front. Mol. Biosci. 2022, 9, 970109. [Google Scholar] [CrossRef] [PubMed]
- Carravetta, V.; Monti, S. Peptide−TiO2 Surface Interaction in Solution by Ab Initio and Molecular Dynamics Simulations. J. Phys. Chem. B 2006, 110, 6160–6169. [Google Scholar] [CrossRef]
- Roussel, G.; Caudano, Y.; Matagne, A.; Sansom, M.S.; Perpète, E.A.; Michaux, C. Peptide-surfactant interactions: A combined spectroscopic and molecular dynamics simulation approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.H.; Berkowitz, M.L. Interaction Between Amyloid-β (1–42) Peptide and Phospholipid Bilayers: A Molecular Dynamics Study. Biophys. J. 2009, 96, 785–797. [Google Scholar] [CrossRef] [PubMed]










| Geometries in Figure 2 | Intramolecular H– Bonds | Intermolecular H– Bonds |
|---|---|---|
| Geometry (e) | 2 | 3 |
| Geometry (f) | 3 | 2 |
| Geometry (g) | 3 | 3 |
| Geometry (h) | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffaini, G.; Pirozzi, P.; Catauro, M.; D’Angelo, A. Hybrid Organic–Inorganic Biomaterials as Drug Delivery Systems: A Molecular Dynamics Study of Quercetin Adsorption on Amorphous Silica Surfaces. Coatings 2024, 14, 234. https://doi.org/10.3390/coatings14020234
Raffaini G, Pirozzi P, Catauro M, D’Angelo A. Hybrid Organic–Inorganic Biomaterials as Drug Delivery Systems: A Molecular Dynamics Study of Quercetin Adsorption on Amorphous Silica Surfaces. Coatings. 2024; 14(2):234. https://doi.org/10.3390/coatings14020234
Chicago/Turabian StyleRaffaini, Giuseppina, Pasqualina Pirozzi, Michelina Catauro, and Antonio D’Angelo. 2024. "Hybrid Organic–Inorganic Biomaterials as Drug Delivery Systems: A Molecular Dynamics Study of Quercetin Adsorption on Amorphous Silica Surfaces" Coatings 14, no. 2: 234. https://doi.org/10.3390/coatings14020234
APA StyleRaffaini, G., Pirozzi, P., Catauro, M., & D’Angelo, A. (2024). Hybrid Organic–Inorganic Biomaterials as Drug Delivery Systems: A Molecular Dynamics Study of Quercetin Adsorption on Amorphous Silica Surfaces. Coatings, 14(2), 234. https://doi.org/10.3390/coatings14020234