Atmospheric-Pressure Plasma Jet Processed Pt-Decorated Reduced Graphene Oxides for Counter-Electrodes of Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Graphene/Pt Pastes
2.2. Preparation of TiO2 Pastes
2.3. Operation Parameters of APPJ
2.4. Assembly of DSSCs
2.5. Characterization of DSSCs and Materials
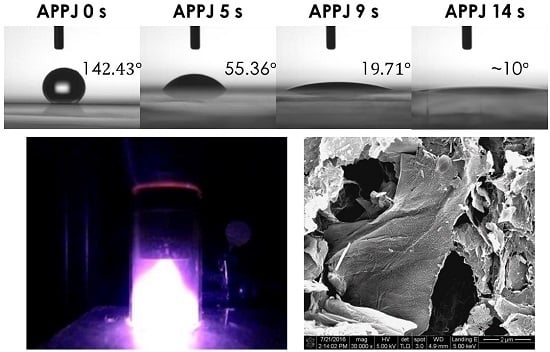
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schutze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Roth, J.R.; Nourgostar, S.; Bonds, T.A. The one atmosphere uniform glow discharge plasma (OAUGDP)-a platform technology for the 21st century. IEEE Trans. Plasma Sci. 2007, 35, 233–250. [Google Scholar] [CrossRef]
- Chen, J.Z.; Hsu, C.C.; Wang, C.; Liao, W.Y.; Wu, C.H.; Wu, T.J.; Liu, H.W.; Chang, H.; Lien, S.T.; Li, H.C.; et al. Rapid atmospheric-pressure-plasma-jet processed porous materials for energy harvesting and storage devices. Coatings 2015, 5, 26–38. [Google Scholar] [CrossRef]
- Babayan, S.; Jeong, J.; Schütze, A.; Tu, V.; Moravej, M.; Selwyn, G.; Hicks, R. Deposition of silicon dioxide films with a non-equilibrium atmospheric-pressure plasma jet. Plasma Sour. Sci. Technol. 2001, 10, 573–578. [Google Scholar] [CrossRef]
- Foest, R.; Kindel, E.; Ohl, A.; Stieber, M.; Weltmann, K.-D. Non-thermal atmospheric pressure discharges for surface modification. Plasma Phy. Control. Fusion 2005, 47, B525–B536. [Google Scholar] [CrossRef]
- Chen, J.Z.; Wang, C.; Hsu, C.C.; CHeng, I.C. Ultrafast synthesis of carbon-nanotube counter-electrodes for dye-sensitized solar cells using an atmospheric-pressure plasma jet. Carbon 2016, 98, 34–40. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, I.C.; Chen, J.Z. Ultrafast atmospheric-pressure-plasma-jet sintering of nanoporous TiO2-SnO2 composites with features defined by screen-printing. ECS J. Solid State Sci. Technol. 2015, 4, 3020–3025. [Google Scholar] [CrossRef]
- Chang, H.; Hsu, C.M.; Kao, P.K.; Yang, Y.J.; Hsu, C.C.; Cheng, I.C.; Chen, J.Z. Dye-sensitized solar cells with nanoporous TiO2 photoanodes sintered by N2 and air atmospheric pressure plasma jets with/without air-quenching. J. Power Sources 2014, 251, 215–221. [Google Scholar] [CrossRef]
- Chen, J.Z.; Liao, W.Y.; Hsieh, W.Y.; Hsu, C.C.; Chen, Y.S. All-vanadium redox flow batteries with graphite felt electrodes treated by atmospheric pressure plasma jets. J. Power Sources 2015, 274, 894–898. [Google Scholar] [CrossRef]
- Chou, C.Y.; Chang, H.M.; Liu, H.W.; Yang, Y.J.; Hsu, C.C.; Cheng, I.C.; Chen, J.Z. Atmospheric-pressure-plasma-jet processed nanoporous TiO2 photoanodes and pt counter-electrodes for dye-sensitized solar cells. RSC Adv. 2015, 5, 45662–45667. [Google Scholar] [CrossRef]
- Barwe, B.; Riedel, F.; Cibulka, O.E.; Pelant, I.; Benedikt, J. Silicon nanoparticle formation depending on the discharge conditions of an atmospheric radio-frequency driven microplasma with argon/silane/hydrogen gases. J. Phys. D Appl. Phys. 2015, 48, 314001. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Jafri, R.I.; Arockiadoss, T.; Ramaprabhu, S. Nanostructured pt decorated graphene and multi walled carbon nanotube based room temperature hydrogen gas sensor. Nanoscale 2009, 1, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of graphene’s properties by reversible hydrogenation: Evidence for graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Ren, W.C.; Jalil, R.; Riaz, I.; Kravets, V.G.; Britnell, L.; Blake, P.; Schedin, F.; Mayorov, A.S.; Yuan, S.J.; et al. Fluorographene: A two-dimensional counterpart of teflon. Small 2010, 6, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Durstock, M.; Dai, L.M. Graphene oxide derivatives as hole and electron-extraction layers for high-performance polymer solar cells. Energy Environ. Sci. 2014, 7, 1297–1306. [Google Scholar] [CrossRef]
- Chartarrayawadee, W.; Moulton, S.E.; Li, D.; Too, C.O.; Wallace, G.G. Novel composite graphene/platinum electro-catalytic electrodes prepared by electrophoretic deposition from colloidal solutions. Electrochim. Acta 2012, 60, 213–223. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Pradeep, K.R.; Raghul, A.; Senthilkumar, R.; Rangarajan, M.; Kothurkar, N.K. One-step synthesis of Pt-decorated graphene-carbon nanotubes for the electrochemical sensing of dopamine, uric acid and ascorbic acid. Anal. Methods 2015, 7, 779–786. [Google Scholar] [CrossRef]
- Wang, J.W.; Rathi, S.; Singh, B.; Lee, I.; Joh, H.I.; Kim, G.H. Alternating current dielectrophoresis optimization of pt-decorated graphene oxide nanostructures for proficient hydrogen gas sensor. ACS Appl. Mater. Interterfaces 2015, 7, 13768–13775. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.D.; Hoa, N.T.Q.; Larina, L.L.; Lee, J.K.; Choi, H.S. Graphene-platinum nanohybrid as a robust and low-cost counter electrode for dye-sensitized solar cells. Nanoscale 2013, 5, 12237–12244. [Google Scholar] [CrossRef] [PubMed]
- Elbohy, H.; Aboagye, A.; Sigdel, S.; Wang, Q.; Sayyad, M.H.; Zhang, L.F.; Qiao, Q.Q. Graphene-embedded carbon nanofibers decorated with pt nanoneedles for high efficiency dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 17721–17727. [Google Scholar] [CrossRef]
- Hoshi, H.; Tanaka, S.; Miyoshi, T. Pt-graphene electrodes for dye-sensitized solar cells. Mater. Sci. Eng. B 2014, 190, 47–51. [Google Scholar] [CrossRef]
- Yeh, M.H.; Lin, L.Y.; Su, J.S.; Leu, Y.A.; Vittal, R.; Sun, C.L.; Ho, K.C. Nanocomposite graphene/Pt electrocatalyst as economical counter electrode for dye-sensitized solar cells. Chemelectrochem 2014, 1, 416–425. [Google Scholar] [CrossRef]
- Liu, H.W.; Liang, S.P.; Wu, T.J.; Chang, H.; Kao, P.K.; Hsu, C.C.; Chen, J.Z.; Chou, P.T.; Cheng, I.C. Rapid atmospheric pressure plasma jet processed reduced graphene oxide counter electrodes for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 15105–15112. [Google Scholar] [CrossRef] [PubMed]








| APPJ Sintered | 0 s | 5 s | 9 s | 14 s |
|---|---|---|---|---|
| 0 μL H2PtCl6 solution | ||||
| Jsc (mA/cm2) | 3.50 | 6.88 | 8.48 | 4.96 |
| Voc (V) | 0.61 | 0.76 | 0.74 | 0.64 |
| FF (%) | 8.76 | 25.03 | 31.14 | 9.96 |
| EFF (%) | 0.19 | 1.31 | 2.01 | 0.32 |
| 25 μL H2PtCl6 solution | ||||
| Jsc (mA/cm2) | 4.39 | 6.32 | 7.72 | 6.31 |
| Voc (V) | 0.63 | 0.72 | 0.74 | 0.75 |
| FF (%) | 8.90 | 29.70 | 58.57 | 56.62 |
| EFF (%) | 0.25 | 1.35 | 3.35 | 2.68 |
| 50 μL H2PtCl6 solution | ||||
| Jsc (mA/cm2) | 5.09 | 8.05 | 8.26 | 6.85 |
| Voc (V) | 0.63 | 0.77 | 0.77 | 0.76 |
| FF (%) | 8.89 | 45.98 | 63.02 | 61.58 |
| EFF (%) | 0.27 | 2.85 | 3.92 | 3.28 |
| 75 μL H2PtCl6 solution | ||||
| Jsc (mA/cm2) | 6.42 | 7.37 | 8.67 | 7.85 |
| Voc (V) | 0.67 | 0.76 | 0.77 | 0.78 |
| FF (%) | 7.91 | 43.18 | 65.73 | 64.25 |
| EFF (%) | 0.34 | 2.42 | 4.39 | 3.93 |
| 100 μL H2PtCl6 solution | ||||
| Jsc (mA/cm2) | 5.02 | 8.37 | 9.20 | 7.97 |
| Voc (V) | 0.72 | 0.76 | 0.79 | 0.78 |
| FF (%) | 8.20 | 50.53 | 65.54 | 64.52 |
| EFF (%) | 0.30 | 3.21 | 4.76 | 4.01 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, T.-H.; Chiu, Y.-F.; Chen, C.-W.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Atmospheric-Pressure Plasma Jet Processed Pt-Decorated Reduced Graphene Oxides for Counter-Electrodes of Dye-Sensitized Solar Cells. Coatings 2016, 6, 44. https://doi.org/10.3390/coatings6040044
Wan T-H, Chiu Y-F, Chen C-W, Hsu C-C, Cheng I-C, Chen J-Z. Atmospheric-Pressure Plasma Jet Processed Pt-Decorated Reduced Graphene Oxides for Counter-Electrodes of Dye-Sensitized Solar Cells. Coatings. 2016; 6(4):44. https://doi.org/10.3390/coatings6040044
Chicago/Turabian StyleWan, Ting-Hao, Yi-Fan Chiu, Chieh-Wen Chen, Cheng-Che Hsu, I-Chun Cheng, and Jian-Zhang Chen. 2016. "Atmospheric-Pressure Plasma Jet Processed Pt-Decorated Reduced Graphene Oxides for Counter-Electrodes of Dye-Sensitized Solar Cells" Coatings 6, no. 4: 44. https://doi.org/10.3390/coatings6040044
APA StyleWan, T. -H., Chiu, Y. -F., Chen, C. -W., Hsu, C. -C., Cheng, I. -C., & Chen, J. -Z. (2016). Atmospheric-Pressure Plasma Jet Processed Pt-Decorated Reduced Graphene Oxides for Counter-Electrodes of Dye-Sensitized Solar Cells. Coatings, 6(4), 44. https://doi.org/10.3390/coatings6040044