Preparation and Properties of High Hardness Ultraviolet Curable Polyethylene Terephthalates Surface Coatings Modified with Octavinyl-Polyhedral Oligomeric Silsesquioxane
Abstract
:1. Introduction
2. Experimental Section
2.1. Material
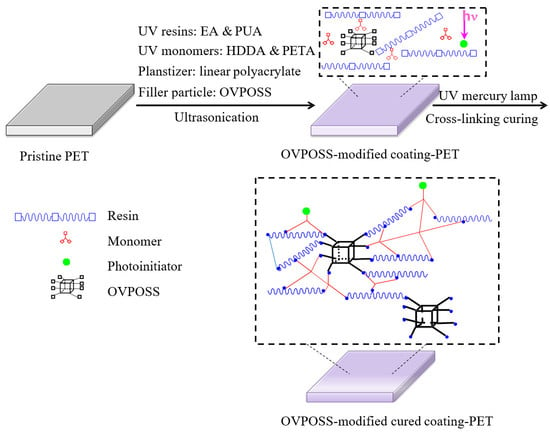
2.2. Preparation of the Nanocomposite Films
2.3. Characterization
3. Results and Discussion
3.1. Formula Optimization of UV-Curable Organic Coatings
3.2. Effects of Nanoparticle Categories on the Harness of Hybrid Coatings
3.3. The Transmittance of Composite Coating
3.4. The Fourier Transform IR (FTIR) Spectroscopy of Composite Coating
3.5. Thermal Characterization
3.6. Dynamic–Mechanical Characterization
3.7. SEM Characterization
3.8. AFM Characterization
4. Properties Comparison
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agnieszka, E.W.; Małgorzata, J.; Agata, G.; Marta, W. Interfacial properties of PET and PET/starch polymers developed by air plasma processing. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 323–331. [Google Scholar]
- Gotoh, K.; Kobayashi, Y.; Yasukawa, A.; Ishigami, Y. Surface modification of PET films by atmospheric pressure plasma exposure with three reactive gas sources. Colloid Polym. Sci. 2012, 290, 1005–1014. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, Z. Influence of low octavinyl-polyhedral oligomeric silsesquioxanes loadings on the crystallization kinetics and morphology of poly(ethylene suberate). Thermochim. Acta 2017, 655, 94–100. [Google Scholar] [CrossRef]
- Ng, Y.-H.; Tay, S.-W.; Liang, H. Donut-like hybrid latex comprising discrete thermoplastic lobe and thermosetting shell. Polymer 2016, 98, 252–262. [Google Scholar] [CrossRef]
- Liao, W.; Huang, X.; Ye, L.; Lan, S.; Fan, H. Synthesis of composite latexes of polyhedral oligomeric silsesquioxane and fluorine containing poly(styrene-acrylate by emulsion copolymerization. J. Appl. Polym. Sci. 2016, 133, 43455. [Google Scholar] [CrossRef]
- Zhang, C.; Bai, X.; Lian, X.; Dou, Y.; Liu, H. Study on morphology and mechanical properties of PMMA-based nanocomposites containing POSS molecules or functionalized SiO2 particles. High Perform. Polym. 2011, 23, 468–476. [Google Scholar] [CrossRef]
- Amerio, E.; Sangermano, M.; Colucci, G.; Malucelli, G.; Messori, M. UV curing of organic–inorganic hybrid coatings containing polyhedral oligomeric silsesquioxane blocks. Macromol. Mater. Eng. 2010, 293, 700–707. [Google Scholar] [CrossRef]
- Akiyama, H.; Ohtani, N. Fabrication and optical characterization of organic–inorganic hybrid films using ultraviolet-curable silsesquioxanes. Jpn. J. Appl. Phys. 2014, 53, 02BC18. [Google Scholar] [CrossRef]
- Lee, A.S.; Jo, Y.Y.; Jeon, H.; Choi, S.S.; Baek, K.Y.; Hwang, S.S. Mechanical properties of thiol-ene UV-curable thermoplastic poly-silsesquioxanes. Polymer 2015, 68, 140–146. [Google Scholar] [CrossRef]
- Jankauskaitė, V.; Lazauskas, A.; Griškonis, E.; Lisauskaitė, A.; Žukienė, K. UV-curable aliphatic silicone acrylate organic–inorganic hybrid coatings with antibacterial activity. Molecules 2017, 22, 964. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Di Pierro, A.; Alongi, J.; Saracco, G. Controlling the melt dripping of polyester fabrics by tuning the ionic strength of polyhedral oligomeric silsesquioxane and sodium montmorillonite coatings assembled through layer by layer. J. Colloid Interface Sci. 2018, 510, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Devaux, E.; Rochery, M.; Bourbigot, S. Polyurethane/clay and polyurethane/POSS nanocomposites as flame retarded coating for polyester and cotton fabrics. Fire Mater. 2002, 26, 149–154. [Google Scholar] [CrossRef]
- Malucelli, G.; Fioravanti, A.; Francioso, L.; De Pascali, C.; Signore, M.A.; Carotta, M.C.; Bonanno, A.; Duraccio, D. Preparation and characterization of UV-cured composite films containing ZnO nanostructures: Effect of filler geometric features on piezoelectric response. Prog. Org. Coat. 2017, 109, 45–54. [Google Scholar] [CrossRef]
- Mishra, K.; Pandey, G.; Singh, R.P. Enhancing the mechanical properties of an epoxy resin using polyhedral oligomeric silsesquioxane (POSS) as nano-reinforcement. Polym. Test. 2017, 62, 210–218. [Google Scholar] [CrossRef]
- Cong, C.; Cui, C.; Meng, X.; Zhou, Q. Structure and property of tetrafluoroethylene-propylene elastomer-OVPOSS composites. J. Appl. Polym. Sci. 2013, 130, 1281–1288. [Google Scholar]
- Zhou, Z.; Zhang, Y.; Zeng, Z.; Zhang, Y. Properties of POSS-filled polypropylene: Comparison of physical blending and reactive blending. J. Appl. Polym. Sci. 2008, 110, 3745–3751. [Google Scholar] [CrossRef]
- Takeuchi, H.; Konno, T.; Mori, H. Synthesis of multifunctional silsesquioxane nanoparticles with hydroxyl and polymerizable groups for UV-curable hybrid coating. React. Funct. Polym. 2017, 115, 43–52. [Google Scholar] [CrossRef]
- Florea, N.M.; Lungu, A.; Badica, P.; Craciun, L.; Enculescu, M.; Ghita, D.G.; Ionescu, C.; Zgirian, R.G.; Iovu, H. Novel nanocomposites based on epoxy resin/epoxy-functionalized polydimethylsiloxane reinforced with POSS. Compos. Part B Eng. 2015, 75, 226–234. [Google Scholar] [CrossRef]
- GB/T 6739-86 Determination of Film Hardness by Pencil Test; National Technical Supervision Bureau: Beijing, China, 1986.
- GB/T 1732-93 Determination of Impact Resistance of Film; National Technical Supervision Bureau: Beijing, China, 1993.
- GB/T 9286-1998 Paints and Varnishes-Cross Cut Test for Films; National Technical Supervision Bureau: Beijing, China, 1998.
- GB/T 6742-86 Bend Test for Coating (Cylindrical Mandrel); National Bureau of Standards: Beijing, China, 1986.
- GB/T 1733-1993 Determination of Resistance to Water of Films; National Technical Supervision Bureau: Beijing, China, 1993.
- GB/T 1763-1979 Methods of Test for Chemical Resistance of Paint Films; National Standards Administration: Beijing, China, 1979.
- Zhao, Y.; Schiraldi, D.A. Thermal and mechanical properties of polyhedral oligomeric silsesquioxane (POSS)/polycarbonate composites. Polymer 2005, 46, 11640–11647. [Google Scholar]
- Chiu, Y.-C.; Chou, I.-C.; Tsai, H.-C.; Riang, L.; Ma, C.-C.M. Morphology, thermal and mechanical properties of the polyhedral oligomeric silsesquioxane side-chain epoxy hybrid material. J. Appl. Polym. Sci. 2010, 118, 3723–3732. [Google Scholar] [CrossRef]
- Eftekhari-Sis, B.; Rahimkhoei, V.; Akbari, A.; Araghi, H.Y. Cubic polyhedral oligomeric silsesquioxane nano-cross-linked hybrid hydrogels: Synthesis, characterization, swelling and dye adsorption properties. React. Funct. Polym. 2018, 128, 47–57. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A. Mono substituted octaphenyl POSSs: The effects of substituents on thermal properties and solubility. Thermochim. Acta 2017, 655, 117–123. [Google Scholar] [CrossRef]







| Runs | EA (wt %) | PUA (wt %) | HDDA (wt %) | PETA (wt %) | Pencil Hardness |
|---|---|---|---|---|---|
| 1 | 30 | 30 | 15 | 15 | F |
| 2 | 40 | 30 | 10 | 10 | F |
| 3 | 40 | 20 | 15 | 15 | F |
| 4 | 45 | 25 | 10 | 10 | F |
| 5 | 50 | 20 | 10 | 10 | H |
| Nanoparticles | Addition Amount of Nanoparticles (wt %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.10 | 0.20 | 0.30 | 0.40 | 0.50 | 1.50 | 2.50 | 3.50 | 4.50 | |
| JH-10 | 2 H | 2 H | 3 H | 3 H | 3 H | 2 H | 3 H | 2 H | 3 H |
| SiO2 | 2 H | 2 H | 3 H | 3 H | 3 H | 2 H | 4 H | 4 H | 4 H |
| ZrO | 2 B | 2 B | F | F | F | H | 3 H | H | 2 H |
| TiO2 | F | F | F | F | F | 3 H | 3 H | 3 H | 3 H |
| Al2O3 | F | F | 3 H | 3 H | 3 H | 3 H | 3 H | 3 H | 3 H |
| OVPOSS | 2 H | 3 H | 4 H | 3 H | 3 H | 3 H | 2 H | 2 H | 2 H |
| Samples | Nano Particles | Pencil Hardness | Impact Resistance (cm) | Adhesion (Level) | Flexibility (mm) |
|---|---|---|---|---|---|
| Film 1 | 0 | H | 80 | 0 | 2 |
| Film 2 | SiO2 | 3 H | 40 | 0 | 2 |
| Film 3 | JH-10 | 3 H | 40 | 0 | 2 |
| Film 4 | Al2O3 | 3 H | 5 | 2 | 4 |
| Film 5 | OVPOSS | 4 H | 102 | 0 | 2 |
| Runs | T−5% (°C) | T−50% (°C) | Tmax (°C) | Rmax (%/min) | Residue Rate at 600 °C (%) |
|---|---|---|---|---|---|
| Film 1 | 315.5 | 429.6 | 457.3 | 10.19 | 5.868 |
| Film 2 | 319.2 | 431.7 | 455.5 | 10.85 | 6.591 |
| Film 4 | 320.2 | 433.5 | 457.2 | 10.56 | 7.446 |
| Film 5 | 335.2 | 437.9 | 459.6 | 10.22 | 8.265 |
| Runs | E′ (Mpa) | E″ (Mpa) | tan δ | Tg (°C) |
|---|---|---|---|---|
| Film 1 | 167.6 | 111.4 | 0.189 | 122.9 |
| Film 2 | 185.5 | 38.3 | 0.204 | 125.6 |
| Film 4 | 254.3 | 50.8 | 0.202 | 125.2 |
| Film 5 | 258.9 | 58.2 | 0.228 | 127.6 |
| Runs | Ra (nm) | RMS (nm) | Rz (nm) | Rz Count |
|---|---|---|---|---|
| Film 1 | 0.843 | 1.148 | 2.551 | 10 |
| Film 2 | 1.072 | 1.424 | 2.843 | 10 |
| Film 4 | 2.279 | 2.606 | 3.845 | 10 |
| Film 5 | 1.370 | 3.877 | 3.804 | 10 |
| Coating Performance | Unmodified Film | OVPOSS Modified Film |
|---|---|---|
| Pencil hardness | H | 4 H |
| Adhesion (level) | 0 | 0 |
| Impact resistance (g cm) | 80 | 102 |
| Flexibility (mm) | 2 | 2 |
| Water resistance | good a | good c |
| Chemical resistance | good b | good d |
| Transmittance (%) | ≥90 | ≥90 |
| Tg (°C) | 100 | 120 |
| 5% loss weight Tem. (°C) | 315.5 | 335.2 |
| 600 °C residual rate (%) | 5.87 | 8.27 |
| Storage modulus (MPa) | 167.6 | 258.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.; Tang, L.; Qu, J. Preparation and Properties of High Hardness Ultraviolet Curable Polyethylene Terephthalates Surface Coatings Modified with Octavinyl-Polyhedral Oligomeric Silsesquioxane. Coatings 2018, 8, 411. https://doi.org/10.3390/coatings8110411
Kang T, Tang L, Qu J. Preparation and Properties of High Hardness Ultraviolet Curable Polyethylene Terephthalates Surface Coatings Modified with Octavinyl-Polyhedral Oligomeric Silsesquioxane. Coatings. 2018; 8(11):411. https://doi.org/10.3390/coatings8110411
Chicago/Turabian StyleKang, Tianmiao, Liuyan Tang, and Jinqing Qu. 2018. "Preparation and Properties of High Hardness Ultraviolet Curable Polyethylene Terephthalates Surface Coatings Modified with Octavinyl-Polyhedral Oligomeric Silsesquioxane" Coatings 8, no. 11: 411. https://doi.org/10.3390/coatings8110411
APA StyleKang, T., Tang, L., & Qu, J. (2018). Preparation and Properties of High Hardness Ultraviolet Curable Polyethylene Terephthalates Surface Coatings Modified with Octavinyl-Polyhedral Oligomeric Silsesquioxane. Coatings, 8(11), 411. https://doi.org/10.3390/coatings8110411