Durable Superomniphobic Surface on Cotton Fabrics via Coating of Silicone Rubber and Fluoropolymers
Abstract
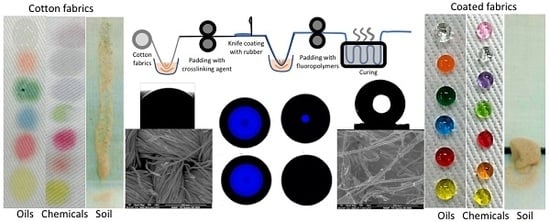
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.3. Characterization and Measurements
2.3.1. Scanning Electron Microscopy
2.3.2. Thickness
2.3.3. FTIR Spectroscopy
2.3.4. Handle
2.3.5. Water Contact Angle
2.3.6. Oil Repellency
2.3.7. Aqueous Liquid Repellency
2.3.8. Chemical Resistance
2.3.9. Soil Resistance
2.3.10. Laundering Test
2.3.11. Water Repellency
2.3.12. Air Permeability
2.3.13. Pilling Resistance
2.3.14. Thermal Resistance
2.3.15. Water Vapor Resistance
2.3.16. Moisture Management Property
3. Results and Discussion
3.1. Characterization
3.1.1. Morphology
3.1.2. Thickness
3.1.3. Handle
3.1.4. FTIR Spectra
3.2. Protection
3.2.1. Water Contact Angle
3.2.2. Water Repellency and Air Permeability
3.2.3. Aqueous Liquid Repellency
3.2.4. Oil Repellency
3.2.5. Chemical Resistance
3.2.6. Soil Resistance
3.3. Comfort
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, S.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. A review on special wettability textiles: Theoretical models, fabrication technologies and multifunctional applications. J. Mater. Chem. 2017, 5, 31–55. [Google Scholar] [CrossRef]
- Owen, M.J.; Dvornic, R. Surface Applications of Silicones, in Silicone Surface Science; Springer: Berlin, Germany, 2012; pp. 355–374. [Google Scholar]
- Xiang, D.; Liu, L.; Liang, Y. Effect of hard segment content on structure, dielectric and mechanical properties of hydroxyl-terminated butadiene-acrylonitrile copolymer-based polyurethane elastomers. Polymer 2017, 132, 180–187. [Google Scholar] [CrossRef]
- Holme, I. Innovative technologies for high performance textiles. Color. Technol. 2007, 123, 59–73. [Google Scholar] [CrossRef]
- Chen, L.; Wu, F.; Li, Y.; Wang, Y.; Si, L.; Lee, K.I.; Fei, B. Robust and elastic superhydrophobic breathable fibrous membrane with in situ grown hierarchical structures. J. Membr. Sci. 2018, 547, 93–98. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Ding, J.; Feng, L.; Wang, X.; Lin, T. Durable, Self-Healing Superhydrophobic and Superoleophobic Surfaces from Fluorinated-Decyl Polyhedral Oligomeric Silsesquioxane and Hydrolyzed Fluorinated Alkyl Silane. Angew. Chem. Int. Ed. 2011, 50, 11433–11436. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wang, H.; Zhou, H.; Lin, T. Self-cleaning, superhydrophobic cotton fabrics with excellent washing durability, solvent resistance and chemical stability prepared from an SU-8 derived surface coating. RSC Adv. 2015, 5, 61044–61050. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Boinovich, L.B.; Bezdomnikov, A.A.; Chulkova, E.V.; Emelyanenko, K.A. Reinforced Superhydrophobic Coating on Silicone Rubber for Longstanding Anti-Icing Performance in Severe Conditions. ACS Appl. Mater. Interfaces 2017, 9, 24210–24219. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Yoo, D.I.; Son, K. Development of thermoregulating textile materials with microencapsulated phase change materials (PCM). IV. Performance properties and hand of fabrics treated with PCM microcapsules. J. Appl. Polym. Sci. 2005, 97, 910–915. [Google Scholar] [CrossRef]
- Lu, X.; Sun, Y.; Chen, Z.; Gao, Y. A multi-functional textile that combines self-cleaning, water-proofing and VO2-based temperature-responsive thermoregulating. Sol. Energy Mater. Sol. Cells 2017, 159, 102–111. [Google Scholar] [CrossRef]
- Pan, S.; Kota, A.K.; Mabry, J.M.; Tuteja, A. Superomniphobic surfaces for effective chemical shielding. J. Am. Chem. Soc. 2012, 135, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, A.R. High Performance textiles for Heat and Fire Protection A2—Lawrence, Carl A. In High Performance Textiles and Their Applications; Elsevier: Cambridge, UK, 2014; pp. 144–175. [Google Scholar]
- Park, Y.; Kim, Y.; Baluch, A.H.; Kim, C.G. Empirical study of the high velocity impact energy absorption characteristics of shear thickening fluid (STF) impregnated Kevlar fabric. Int. J. Impact Eng. 2014, 72, 67–74. [Google Scholar] [CrossRef]
- Thakare, V.B.; Tripathi, N.K.; Singh, V.V.; Sathe, M.; Singh, B. Activated Carbon Fabric: An Adsorbent Material for Chemical Protective Clothing. Def. Sci. J. 2017, 68, 83–90. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Wang, X.; Lin, T. Superhydrophobic nanofibre membranes: Effects of particulate coating on hydrophobicity and surface properties. J. Text. Inst. 2012, 103, 937–944. [Google Scholar] [CrossRef]
- Tan, S.; Li, J.; Gao, G.; Li, H.; Zhang, Z. Synthesis of fluoropolymer containing tunable unsaturation by a controlled dehydrochlorination of P (VDF-co-CTFE) and its curing for high performance rubber applications. J. Mater. Chem. 2012, 22, 18496–18504. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W. Preparation of hydrophobic metal-organic frameworks via plasma enhanced chemical vapor deposition of perfluoroalkanes for the removal of ammonia. J. Vis. Exp. 2013, 80, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Satam, D.; Lee, H.J.; Wilusz, E. An approach to mass customization of military uniforms using superoleophobic nonwoven fabrics. AATCC Rev. 2010, 10, 59–63. [Google Scholar]
- Singh, A.K.; Singh, J.K. Fabrication of durable superhydrophobic coatings on cotton fabrics with photocatalytic activity by fluorine-free chemical modification for dual-functional water purification. New J. Chem. 2017, 41, 4618–4628. [Google Scholar] [CrossRef]
- Moiz, A.; Vijayan, A.; Padhye, R.; Wang, X. Chemical and water protective surface on cotton fabric by pad-knife-pad coating of WPU-PDMS-TMS. Cellulose 2016, 23, 3377–3388. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, C.; Ye, W.; Wang, H.; Feng, D.; Li, Q.; Huan, B. A facile dip-coating approach to prepare SiO2/fluoropolymer coating for superhydrophobic and superoleophobic fabrics with self-cleaning property. J. Appl. Polym. Sci. 2015, 132, 41458. [Google Scholar] [CrossRef]
- Kang, Y.K.; Park, C.H.; Kim, J.; Kang, T.J. Application of electrospun polyurethane web to breathable water-proof fabrics. Fibers Polym. 2007, 8, 564–570. [Google Scholar] [CrossRef]
- Schutzius, T.M.; Bayer, I.S.; Tiwari, M.K.; Megaridis, C.M. Novel Fluoropolymer Blends for the Fabrication of Sprayable Multifunctional Superhydrophobic Nanostructured Composites. Ind. Eng. Chem. Res. 2011, 50, 11117–11123. [Google Scholar] [CrossRef]
- Nouri, N.M.; Saadat-Bakhsh, M. Fabrication method of large-scale and mechanically durable superhydrophobic silicon rubber/aerogel coating on fibrous substrates. J. Coat. Technol. Res. 2017, 14, 477–488. [Google Scholar] [CrossRef]
- Seitz, V.; Arzt, K.; Mahnel, S.; Rapp, C.; Schwaminger, S.; Hoffstetter, M.; Wintermantel, E. Improvement of adhesion strength of self-adhesive silicone rubber on thermoplastic substrates–Comparison of an atmospheric pressure plasma jet (APPJ) and a Pyrosil® flame. Int. J. Adhes. Adhes. 2016, 66, 65–72. [Google Scholar] [CrossRef]
- Kang, E.T.; Tan, K.L.; Kato, K.; Uyama, Y.; Ikada, Y. Surface Modification and Functionalization of Polytetrafluoroethylene Films. Macromolecules 1996, 29, 6872–6879. [Google Scholar] [CrossRef]
- Hekster, F.M.; De Voogt, P.; Pijnenburg, A.M.C.M.; Laane, R.W.P.M. Perfluoroalkylated Substances: Aquatic Environmental Assessment; University of Amsterdam: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Haile, M.; Fincher, C.; Fomete, S.; Grunlan, J.C. Water-soluble polyelectrolyte complexes that extinguish fire on cotton fabric when deposited as pH-cured nanocoating. Polym. Degrad. Stab. 2015, 114, 60–64. [Google Scholar] [CrossRef]
- Carsio, F.; Alongi, J. Few durable layers suppress cotton comustion due to the joint combination of layer by layer assembly and UV-curing. RSC Adv. 2015, 5, 71482–71490. [Google Scholar] [CrossRef]
- Alongi, F.; Carsio, F. All-inorganic intumescent nanocoating containing montmorillonite nanoplatelets in ammonium polyphosphate matrix capable of preventing cotton ignition. Polymers 2016, 8, 430. [Google Scholar] [CrossRef]
- Haile, M.; Leistner, M.; Sarwar, O.; Toler, C.M.; Henderson, R.; Grunlan, J.C. A wash-durable polyelectrolyte complex that extinguishes flames on polyester-cotton fabric. RSC Adv. 2016, 6, 33998–34004. [Google Scholar] [CrossRef]
- Moiz, A.; Padhye, R.; Wang, X. Coating of TPU-PDMS-TMS on Polycotton Fabrics for Versatile Protection. Polymers 2017, 9, 660. [Google Scholar] [CrossRef]
- Dhiman, G.; Chakraborty, J.N. Soil release performance of cotton finished with oleophobol CPR and CMC-Na salt. Fash. Text. 2014, 1, 23. [Google Scholar] [CrossRef]
- Yüksekkaya, M.E.; Howard, T.; Adanu, S. Influence of the fabric properties on fabric stiffness for the industrial fabrics. J. Text. Appar. Tekst. Konfeksiyon 2008, 18, 263–267. [Google Scholar]
- Ye, W.; Xin, J.H.; Li, P.; Lee, K.L.D.; Kwong, T.L. Durable antibacterial finish on cotton fabric by using chitosan-based polymeric core-shell particles. J. Appl. Polym. Sci. 2006, 102, 1787–1793. [Google Scholar] [CrossRef]
- Baek, D.K.; Khonsari, M.M. Fretting behavior of a rubber coating: Effect of temperature and surface roughness variations. Wear 2008, 265, 620–625. [Google Scholar] [CrossRef]
- Jassal, M.; Khungar, A.; Bajaj, P.; Sinha, T.J.M. Waterproof breathable polymeric coatings based on polyurethanes. J. Ind. Text. 2004, 33, 269–280. [Google Scholar] [CrossRef]
- Vazquez, F. Silicone Softeners for Stain Repellent and Stain Release Fabric Finishing; Dow Corning Corporation: Midland, MI, USA, 2005. [Google Scholar]
- Jarvis, N.; Zisman, W. Surface Chemistry of Fluorochemicals; U.S. Naval Research Laboratory: Washington, DC, USA, 1965; pp. 1–34. [Google Scholar]
- Lee, L.-H. Wettability and conformation of reactive polysiloxanes. J. Colloid Interface Sci. 1968, 27, 751–760. [Google Scholar] [CrossRef]
- Liang, L.; Ruckenstein, E. Pervaporation of ethanol-water mixtures through polydimethylsiloxane-polystyrene interpenetrating polymer network supported membranes. J. Membr. Sci. 1996, 114, 227–234. [Google Scholar] [CrossRef]
- Li, N.; Zeng, F.; Wang, Y.; Qu, D.; Hu, W.; Luan, Y.; Dong, S.; Zhang, J.; Bai, Y. Fluorinated polyurethane based on liquid fluorine elastomer (LFH) synthesis via two-step method: The critical value of thermal resistance and mechanical properties. RSC Adv. 2017, 7, 30970–30978. [Google Scholar] [CrossRef]
- Rasid, H.M.; Azhar, N.H.A.; Yusoff, S.F.M. Physicochemical properties of liquid natural rubber bearing fluoro groups for hydrophobic surfaces. J. Polym. Res. 2017, 24, 106. [Google Scholar] [CrossRef]





| Chemicals | Samples | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Acetic acids | 0 | 600 | 600 | 600 | 600 | 600 | 600 | 600 |
| Paraffin oil | 0 | 600 | 600 | 600 | 600 | 600 | 600 | 600 |
| Sodium hydroxide | 0 | 600 | 600 | 600 | 600 | 600 | 600 | 600 |
| n-hexadecane | 10 | 600 | 600 | 600 | 600 | 600 | 600 | 600 |
| Isopropyl alcohol | 0 | 600 | 600 | 600 | 600 | 600 | 600 | 600 |
| Castor oil | 0 | 600 | 600 | 600 | 600 | 600 | 600 | 600 |
| 1,4 Butadiene | 0 | 600 | 600 | 600 | 600 | 600 | 600 | 600 |
| Dimethylformamide | 0 | 600 | 600 | 600 | 600 | 600 | 300 | 600 |
| Acetonitrile | 0 | 600 | 600 | 600 | 600 | 600 | 600 | 600 |
| n-decane | 0 | 0 | 600 | 600 | 600 | 600 | 600 | 600 |
| Methanol | 0 | 10 | 600 | 600 | 600 | 186 | 570 | 600 |
| Sulphuric acids | 0 | 0 | 103 | 105 | 107 | 600 | 600 | 540 |
| n-heptane | 0 | 0 | 39 | 540 | 600 | 120 | 147 | 600 |
| Triethylamine | 0 | 10 | 535 | 26 | 10 | 540 | 23 | 45 |
| Toluene | 0 | 0 | 600 | 0 | 0 | 600 | 0 | 0 |
| Dichloromethane | 0 | 0 | 0 | 0 | 0 | 60 | 10 | 15 |
| n-hexane | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 20 |
| Tetrahydrofuran | 0 | 0 | 0 | 0 | 0 | 18 | 0 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moiz, A.; Padhye, R.; Wang, X. Durable Superomniphobic Surface on Cotton Fabrics via Coating of Silicone Rubber and Fluoropolymers. Coatings 2018, 8, 104. https://doi.org/10.3390/coatings8030104
Moiz A, Padhye R, Wang X. Durable Superomniphobic Surface on Cotton Fabrics via Coating of Silicone Rubber and Fluoropolymers. Coatings. 2018; 8(3):104. https://doi.org/10.3390/coatings8030104
Chicago/Turabian StyleMoiz, Arsheen, Rajiv Padhye, and Xin Wang. 2018. "Durable Superomniphobic Surface on Cotton Fabrics via Coating of Silicone Rubber and Fluoropolymers" Coatings 8, no. 3: 104. https://doi.org/10.3390/coatings8030104
APA StyleMoiz, A., Padhye, R., & Wang, X. (2018). Durable Superomniphobic Surface on Cotton Fabrics via Coating of Silicone Rubber and Fluoropolymers. Coatings, 8(3), 104. https://doi.org/10.3390/coatings8030104