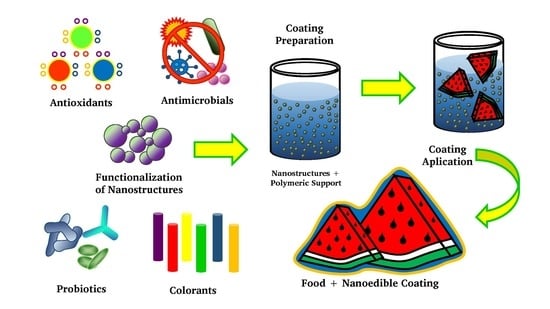
The Functionalization of Nanostructures and Their Potential Applications in Edible Coatings
Abstract
:1. Introduction
2. Nanostructured Matrices
2.1. Polysaccharides
2.2. Lipids
2.3. Proteins
3. Active Substances in the Functionalization of Nanostructures
3.1. Antioxidants
3.2. Antimicrobials
3.3. Probiotic and Pre-Biotic
3.4. Colorants and Flavors
4. Effect of the Functionalization of Nanostructures in Edible Coatings
5. Release of the Active Substances in Edible Coatings
6. Conclusions and Future Trends
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviation
| CG | Cashew gum |
| CMC | Carboxymethyl cellulose |
| CNC | Cellulose nanocrystals |
| CS | Chitosan |
| L-NVs | Lipid-based nanovesicles |
| NCs | Nanocapsules |
| NGs | Nanogels |
| NLC | Nanolipid carrier |
| NPs | Nanoparticles |
| NSs | Niosomes |
| PCL | Poly-ε-caprolactone |
| PEG | Polyethylene glycol |
| PLGA | Polylactide-co-glycolide |
| SLN | Solid Lipid Nanoparticles |
| TG | Tragacanth gum |
References
- Rahman, M.S. Hurdle Technology in Food Preservation. In Minimally Processed Foods: Technologies for Safety, Quality, and Convenience; Siddiqui, M.W., Rahman, M.S., Eds.; Springer: Heidelberg, Germany, 2015; pp. 17–34. [Google Scholar]
- Embuscado, M.E. Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; ISBN 978-0-387-92823-4. [Google Scholar]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Ferreira, G.; Hernandez-Martinez, A.R.; Pool, H.; Molina, G.; Cruz-Soto, M.; Luna-Barcenas, G.; Estevez, M. Synthesis and functionalization of silica-based nanoparticles with fluorescent biocompounds extracted from Eysenhardtia polystachya for biological applications. Mater. Sci. Eng. C 2015, 57, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Gutiérrez-Cortez, E.; Castaño-Tostado, E.; Quintanar-Guerrero, D. Optimization of nanocapsules preparation by the emulsion-diffusion method for food applications. LWT Food Sci. Technol. 2011, 44, 1362–1368. [Google Scholar] [CrossRef]
- Pathakoti, K.; Manubolu, M.; Hwang, H.-M. Nanostructures: Current uses and future applications in food science. J. Food Drug Anal. 2017, 25, 245–253. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hwang, H.M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Monty, J.; Linhardt, R.J. Polysaccharide-based nanocomposites and their applications. Carbohydr. Res. 2015, 405, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, S.; Zheng, H.; Dong, H.; Liu, J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr. Polym. 2015, 123, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Zafar, R.; Zia, K.M.; Tabasum, S.; Jabeen, F.; Noreen, A.; Zuber, M. Polysaccharide based bionanocomposites, properties and applications: A review. Int. J. Biol. Macromol. 2016, 92, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.R.; Pereira, A.; Castro, P.M.; Pintado, M. Production of antimicrobial chitosan nanoparticles against food pathogens. J. Food Eng. 2015, 167, 210–216. [Google Scholar] [CrossRef]
- Soumya, R.S.; Ghosh, S.; Abraham, E.T. Preparation and characterization of guar gum nanoparticles. Int. J. Biol. Macromol. 2010, 46, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Gardesh, A.S.K.; Badii, F.; Hashemi, M.; Ardakani, A.Y.; Maftoonazad, N.; Gorji, A.M. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. LWT Food Sci. Technol. 2016, 70, 33–40. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of food grade nanostructured lipid carrier (NLC) for potential applications in medicinal-functional foods. J. Drug Deliv. Sci. Technol. 2017, 39, 50–58. [Google Scholar] [CrossRef]
- Katouzian, I.; Faridi Esfanjani, A.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Weiss, J.; Decker, E.A.; McClements, D.J.; Kristbergsson, K.; Helgason, T.; Awad, T. Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophys. 2008, 3, 146–154. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.; Mercado-Silva, E.; Ramirez-Zamorano, P.; Cornejo-Villegas, M.A.; Gutiérrez-Cortez, E.; Quintanar-Guerrero, D. Use of solid lipid nanoparticles (SLNs) in edible coatings to increase guava (Psidium guajava L.) shelf-life. Food Res. Int. 2013, 51, 946–953. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Righeschi, C.; Bergonzi, M.C.; Isacchi, B.; Bazzicalupi, C.; Gratteri, P.; Bilia, A.R. Enhanced curcumin permeability by SLN formulation: The PAMPA approach. LWT Food Sci. Technol. 2016, 66, 475–483. [Google Scholar] [CrossRef]
- Tian, H.; Lu, Z.; Li, D.; Hu, J. Preparation and characterization of citral-loaded solid lipid nanoparticles. Food Chem. 2018, 248, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res. Int. 2018, 105, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Keivani Nahr, F.; Ghanbarzadeh, B.; Hamishehkar, H.; Samadi Kafil, H. Food grade nanostructured lipid carrier for cardamom essential oil: Preparation, characterization and antimicrobial activity. J. Funct. Foods 2018, 40, 1–8. [Google Scholar] [CrossRef]
- Raynes, J.K.; Carver, J.A.; Gras, S.L.; Gerrard, J.A. Protein nanostructures in food—Should we be worried? Trends Food Sci. Technol. 2014, 37, 42–50. [Google Scholar] [CrossRef]
- Foegeding, E.A. Food biophysics of protein gels: A challenge of nano and macroscopic proportions. Food Biophys. 2006, 1, 41–50. [Google Scholar] [CrossRef]
- Metwally, A.K.A.; El-Ahmady, S.H.; Hathout, R.M. Selecting optimum protein nano-carriers for natural polyphenols using chemoinformatics tools. Phytomedicine 2016, 23, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Velikov, K.P. Zein as a source of functional colloidal nano- and microstructures. Curr. Opin. Colloid Interface Sci. 2014, 19, 450–458. [Google Scholar] [CrossRef]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Feng, Z.; Wu, G.; Liu, C.; Li, D.; Jiang, B.; Zhang, X. Edible coating based on whey protein isolate nanofibrils for antioxidation and inhibition of product browning. Food Hydrocoll. 2018, 79, 179–188. [Google Scholar] [CrossRef]
- Shahidi, F. Antioxidants in food and food antioxidants. Mol. Nutr. Food Res. 2000, 44, 158–163. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Kumar, S. Free Radicals and Antioxidants: Human and Food System. Adv. Appl. Sci. Res. 2011, 2, 129–135. [Google Scholar]
- Bai, J.-J.; Lee, J.-G.; Lee, S.-Y.; Kim, S.; Choi, M.-J.; Cho, Y. Changes in quality characteristics of pork patties containing antioxidative fish skin peptide or fish skin peptideloaded nanoliposomes during refrigerated storage. Korean J. Food Sci. Anim. Resour. 2017, 37, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Holban, A.M. Impact of Nanoscience in the Food Industry, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupea, A.X.; Pop, M.; Cacig, S. Structure-radical scavenging activity relationships of flavonoids from Ziziphus and Hydrangea extracts. Rev. Chim. 2008, 59, 309–313. [Google Scholar]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Ye, F.; Astete, C.E.; Sabliov, C.M. Entrapment and delivery of α-tocopherol by a self-assembled, alginate-conjugated prodrug nanostructure. Food Hydrocoll. 2017, 72, 62–72. [Google Scholar] [CrossRef]
- Akhoond Zardini, A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2018, 55, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, D.; Sun, C.; McClements, D.J.; Gao, Y. Utilization of interfacial engineering to improve physicochemical stability of β-carotene emulsions: Multilayer coatings formed using protein and protein–polyphenol conjugates. Food Chem. 2016, 205, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, X.; Gong, Y.; Xiao, H.; Julian, D.; Hu, K. Enhancement of curcumin water dispersibility and antioxidant activity using core—Shell protein—Polysaccharide nanoparticles. Food Res. Int. 2016, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of dietary flavonoid fisetin: Formulation and in vitro antioxidant and alpha-glucosidase inhibition activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Swift, S.; Ray, S.; Gizdavic-Nikolaidis, M.; Jin, J.; Perera, C.O. Evaluation of gallic acid loaded zein sub-micron electrospun fibre mats as novel active packaging materials. Food Chem. 2013, 141, 3192–3200. [Google Scholar]
- El-Gogary, R.I.; Rubio, N.; Wang, J.T.W.; Al-Jamal, W.T.; Bourgognon, M.; Kafa, H.; Naeem, M.; Klippstein, R.; Abbate, V.; Leroux, F.; et al. Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano 2014, 8, 1384–1401. [Google Scholar] [CrossRef] [PubMed]
- Aceituno-Medina, M.; Mendoza, S.; Rodríguez, B.A.; Lagaron, J.M.; López-Rubio, A. Improved antioxidant capacity of quercetin and ferulic acid during in-vitro digestion through encapsulation within food-grade electrospun fibers. J. Funct. Foods 2015, 12, 332–341. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, D.; Kumari, A.; Yadav, S.K. Encapsulation of catechin and epicatechin on BSA NPS improved their stability and antioxidant potential. EXCLI J. 2014, 13, 331–346. [Google Scholar] [PubMed]
- Sanna, V.; Lubinu, G.; Madau, P.; Pala, N.; Nurra, S.; Mariani, A.; Sechi, M. Polymeric nanoparticles encapsulating white tea extract for nutraceutical application. J. Agric. Food Chem. 2015, 63, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. Food Sci. Technol. 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Abreu, F.O.M.S.; Oliveira, E.F.; Paula, H.C.B.; Paula, R.C.M. De Chitosan/cashew gum nanogels for essential oil encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Bossio, O.; Gómez-Mascaraque, L.G.; Fernández-Gutiérrez, M.; Vázquez-Lasa, B.; Román, J.S. Amphiphilic polysaccharide nanocarriers with antioxidant properties. J. Bioact. Compat. Polym. 2014, 29, 589–606. [Google Scholar] [CrossRef]
- Ding, X.; Yao, P. Soy Protein/soy polysaccharide complex nanogels: folic acid loading, protection, and controlled delivery. Langmuir 2013, 29, 8636–8644. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Impact of lipid nanoparticle physical state on particle aggregation and {Œ}\le-carotene degradation: Potential limitations of solid lipid nanoparticles. Food Res. Int. 2013, 52, 342–349. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. LWT—food science and technology nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.J.; Huang, C. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Y.; Xie, J.; Wang, X. Effects of combined treatment of electrolysed water and chitosan on the quality attributes and myofibril degradation in farmed obscure puffer fish (Takifugu obscurus) during refrigerated storage. Food Chem. 2011, 129, 1660–1666. [Google Scholar] [CrossRef]
- Kayaci, F.; Umu, O.C.O.; Tekinay, T.; Uyar, T. Antibacterial electrospun poly(lactic acid) (PLA) nano fi brous webs incorporating triclosan/cyclodextrin inclusion complexes. J. Agric. Food Chem. 2013, 61, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: II. Application in bio-based plastics for active packaging. Carbohydr. Polym. 2013, 96, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.; Sharaf, S.; El-Hady, M.M.A.; Hebeish, A. Synthesis and characterization of novel carboxymethylcellulose hydrogels and carboxymethylcellulolse-hydrogel-ZnO-nanocomposites. Carbohydr. Polym. 2013, 95, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Chand, N.; Chaurasia, V. Investigation of water vapor permebility and antimicrobial property of zinc oxide nanoparticles-loaded chitosan-based edible Film. J. Appl. Polym. Sci. 2010, 115, 647–683. [Google Scholar] [CrossRef]
- Kopermsub, P.; Mayen, V.; Warin, C. Nanoencapsulation of nisin and ethylenediaminetetraacetic acid in niosomes and their antibacterial activity. J. Sci. Res. 2012, 4, 457. [Google Scholar] [CrossRef]
- Prombutara, P.; Kulwatthanasal, Y.; Supaka, N.; Sramala, I. Production of nisin-loaded solid lipid nanoparticles for sustained antimicrobial activity. Food Control 2012, 24, 184–190. [Google Scholar] [CrossRef]
- Balcão, V.M.; Costa, C.I.; Matos, C.M.; Moutinho, C.G.; Amorim, M.; Pintado, M.E.; Gomes, A.P.; Vila, M.M.; Teixeira, J.A. Food hydrocolloids nanoencapsulation of bovine lactoferrin for food and biopharmaceutical applications. Food Hydrocoll. 2013, 32, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Poly (DL-lactide-co-glycolide) (PLGA) nanoparticles with entrapped trans-cinnamaldehyde and eugenol for antimicrobial delivery applications. J. Food Sci. 2011, 76, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Liolios, C.C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Morsy, M.K. Stability of antimicrobial activity of pullulan edible films incorporated with nanoparticles and essential oils and their impact on turkey deli meat quality. J. Food Dairy Sci. 2013, 4, 557–573. [Google Scholar]
- Yang, F.L.; Li, X.G.; Zhu, F.; Lei, C.L. Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Agric. Food Chem. 2009, 57, 10156–10162. [Google Scholar] [CrossRef] [PubMed]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef] [PubMed]
- Ghayempour, S.; Montazer, M.; Mahmoudi Rad, M. Tragacanth gum as a natural polymeric wall for producing antimicrobial nanocapsules loaded with plant extract. Int. J. Biol. Macromol. 2015, 81, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.A.; Roy, T.; D’Adamo, C.R.; Wieland, L.S. Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration. Nutrition 2018, 45, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ziemer, C.J.; Gibson, G.R. An overview of probiotics, prebiotics and synbiotics in the functional food concept: Perspectives and future strategies. Int. Dairy J. 1998, 8, 473–479. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Batista, R.A.; Azeredo, H.M.C.; Otoni, C.G. Probiotics and their potential applications in active edible films and coatings. Food Res. Int. 2016, 90, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Probiotics and prebiotics—Progress and challenges. Int. Dairy J. 2008, 18, 969–975. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Floch, M.H. The role of prebiotics and probiotics in gastrointestinal disease. Gastroenterol. Clin. N. Am. 2018, 47, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Charalampopoulos, D.; Rastall, R.A. Prebiotics in foods. Curr. Opin. Biotechnol. 2012, 23, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, T.P.; Mladenovska, K.; Zhivikj, Z.; Pavlova, M.J.; Gjurovski, I.; Ristoski, T.; Petrushevska-Tozi, L. Synbiotic loaded chitosan-Ca-alginate microparticles reduces inflammation in the TNBS model of rat colitis. Int. J. Pharm. 2017, 527, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Sultana, K.; Godward, G.; Reynolds, N.; Arumugaswamy, R.; Peiris, P.; Kailasapathy, K. Encapsulation of probiotic bacteria with alginate–starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int. J. Food Microbiol. 2000, 62, 47–55. [Google Scholar] [CrossRef]
- Ahmad, B.; Gani, A.; Gani, A.; Shah, A.; Ahmad, F. Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018, 239, 287–294. [Google Scholar] [CrossRef]
- Sathyabama, S.; Ranjith, M.; Bruntha, P.; Vijayabharathi, R.; Brindha, V. LWT—Food science and technology co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT Food Sci. Technol. 2014, 57, 419–425. [Google Scholar] [CrossRef]
- De Prisco, A.; Mauriello, G. Probiotication of foods: A focus on microencapsulation tool. Trends Food Sci. Technol. 2016, 48, 27–39. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival During Gastric Transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamy, S.; Bruntha, P. Food bioscience recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods—A review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otoni, C.G.; Espitia, P.J.P.; Avena-Bustillos, R.J.; Mchugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. FRIN 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Tapia, M.S.; Rojas-Graü, M.A.; Rodríguez, F.J.; Ramírez, J.; Carmona, A.; Martin-Belloso, O. Alginate- and Gellan-Based Edible Films for Probiotic Coatings on Fresh-Cut Fruits. J. Food Sci. 2007, 72, E190–E196. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Shojaee-Aliabadi, S.; Koushki, M.R. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT Food Sci. Technol. 2018, 87, 54–60. [Google Scholar] [CrossRef]
- Pereira, J.O.; Soares, J.; Monteiro, M.J.P.; Gomes, A.; Pintado, M. Impact of whey protein coating incorporated with Bi fi dobacterium and Lactobacillus on sliced ham properties. Meat Sci. 2018, 139, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Li, X.Y.; Liu, B.J.; Meng, X.H. Microencapsulation of lactobacillus bulgaricus and survival assays under simulated gastrointestinal conditions. J. Funct. Foods 2017, 29, 248–255. [Google Scholar] [CrossRef]
- Khorasani, A.C.; Shojaosadati, S.A. Pectin-non-starch nanofibers biocomposites as novel gastrointestinal-resistant prebiotics. Int. J. Biol. Macromol. 2017, 94, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Oregel-Zamudio, E.; Angoa-Pérez, M.V.; Oyoque-Salcedo, G.; Aguilar-González, C.N.; Mena-violante, H.G. scientia horticulturae effect of candelilla wax edible coatings combined with biocontrol bacteria on strawberry quality during the shelf-life. Sci. Hortic. 2017, 214, 273–279. [Google Scholar] [CrossRef]
- Dafe, A.; Etemadi, H.; Dilmaghani, A.; Reza, G. International journal of biological macromolecules investigation of pectin/starch hydrogel as a carrier for oral delivery of probiotic bacteria. Int. J. Biol. Macromol. 2017, 97, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.C.; Shojaosadati, S.A. Bacterial nanocellulose-pectin bionanocomposites as prebiotics against drying and gastrointestinal condition. Int. J. Biol. Macromol. 2016, 83, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mascaraque, L.G.; Morfin, R.C.; Pérez-Masiá, R.; Sanchez, G.; Lopez-Rubio, A. Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT Food Sci. Technol. 2016, 69, 438–446. [Google Scholar] [CrossRef]
- Darjani, P.; Hosseini, M.; Kadkhodaee, R.; Milani, E. LWT—Food science and technology in fluence of prebiotic and coating materials on morphology and survival of a probiotic strain of Lactobacillus casei exposed to simulated gastrointestinal conditions. LWT Food Sci. Technol. 2016, 73, 162–167. [Google Scholar] [CrossRef]
- Bersaneti, G.T.; Mantovan, J.; Magri, A.; Antonia, M.; Colabone, P. Edible films based on cassava starch and fructooligosaccharides produced by Bacillus subtilis natto CCT 7712. Carbohydr. Polym. 2016, 151, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Odila Pereira, J.; Soares, J.; Sousa, S.; Madureira, A.R.; Gomes, A.; Pintado, M. Edible films as carrier for lactic acid bacteria. LWT Food Sci. Technol. 2016, 73, 543–550. [Google Scholar] [CrossRef]
- Soukoulis, C.; Singh, P.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Food hydrocolloids compositional and physicochemical factors governing the viability of Lactobacillus rhamnosus GG embedded in starch-protein based edible films. Food Hydrocoll. 2016, 52, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Tavera-Quiroz, M.J.; Romano, N.; Mobili, P.; Pinotti, A.; Gómez-Zavaglia, A.; Bertola, N. Green apple baked snacks functionalized with edible coatings of methylcellulose containing Lactobacillus plantarum. J. Funct. Foods 2015, 16, 164–173. [Google Scholar] [CrossRef]
- Piermaria, J.; Diosma, G.; Aquino, C.; Garrote, G.; Abraham, A. Edible kefiran films as vehicle for probiotic microorganisms. Innov. Food Sci. Emerg. Technol. 2015, 32, 193–199. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG in prebiotic edible films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Yonekura, L.; Gan, H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Food hydrocolloids probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Tavera-Quiroz, M.J.; Bertola, N.; Mobili, P.; Pinotti, A.; Gómez-Zavaglia, A. Edible methylcellulose-based films containing fructo-oligosaccharides as vehicles for lactic acid bacteria. Food Res. Int. 2014, 64, 560–566. [Google Scholar] [CrossRef]
- Kandansamy, K.; Somasundaram, P.D. Microencapsulation of colors by spray drying-A review microencapsulation of colors by spray drying—A review. Int. J. Food Eng. 2012, 8, 1556–3758. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Özkan, G.; Bilek, S.E. Microencapsulation of natural food colourants Applied to Natural Food Colourants. Int. J. Nutr. Food Sci. 2014, 3, 145–156. [Google Scholar] [CrossRef]
- Ozdemir, N.; Pola, C.C.; Teixeira, B.N.; Hill, L.E.; Bayrak, A.; Gomes, C.L. Preparation of black pepper oleoresin inclusion complexes based on beta-cyclodextrin for antioxidant and antimicrobial delivery applications using kneading and freeze drying methods: A comparative study. LWT Food Sci. Technol. 2018, 91, 439–445. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Quintanar-Guerrero, D.; Del Real-López, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, M.L. Effect of sucrose concentration and pH onto the physical stability of β-carotene nanocapsules. LWT 2018, 90, 354–361. [Google Scholar] [CrossRef]
- Lu, M.; Li, Z.; Liang, H.; Shi, M.; Zhao, L.; Li, W.; Chen, Y.; Wu, J.; Wang, S.; Chen, X.; et al. Controlled release of anthocyanins from oxidized konjac glucomannan microspheres stabilized by chitosan oligosaccharides. Food Hydrocolld. 2015, 51, 476–485. [Google Scholar] [CrossRef]
- Ravanfar, R.; Tamaddon, A.M.; Niakousari, M.; Moein, M.R. Preservation of anthocyanins in solid lipid nanoparticles: Optimization of a microemulsion dilution method using the Placket-Burman and Box-Behnken designs. Food Chem. 2016, 199, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.P.; Paese, K.; Guterres, S.S.; Pohlmann, A.R.; Jablonski, A.; Flôres, S.H.; de Oliveira Rios, A. Stability study of lycopene-loaded lipid-core nanocapsules under temperature and photosensitization. LWT Food Sci. Technol. 2016, 71, 190–195. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, J.F. The release kinetics of β-carotene nanocapsules/xanthan gum coating and quality changes in fresh-cut melon (cantaloupe). Carbohydr. Polym. 2017, 157. [Google Scholar] [CrossRef] [PubMed]
- Ersus Bilek, S.; Yılmaz, F.M.; Özkan, G. The effects of industrial production on black carrot concentrate quality and encapsulation of anthocyanins in whey protein hydrogels. Food Bioprod. Process. 2017, 102, 72–80. [Google Scholar] [CrossRef]
- Wang, M.; Fu, Y.; Chen, G.; Shi, Y.; Li, X.; Zhang, H.; Shen, Y. Fabrication and characterization of carboxymethyl chitosan and tea polyphenols coating on zein nanoparticles to encapsulate β-carotene by anti-solvent precipitation method. Food Hydrocolld. 2017, 77, 577–587. [Google Scholar] [CrossRef]
- Atay, E.; Fabra, M.J.; Martínez-Sanz, M.; Gomez-Mascaraque, L.G.; Altan, A.; Lopez-Rubio, A. Development and characterization of chitosan/gelatin electrosprayed microparticles as food grade delivery vehicles for anthocyanin extracts. Food Hydrocoll. 2017, 77, 699–710. [Google Scholar] [CrossRef]
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional materials in food nanotechnology. J. Food Sci. 2006, 71, R107–R116. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; van Reenen, A.J.; Opara, U.L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT Food Sci. Technol. 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Tampau, A.; González-Martinez, C.; Chiralt, A. Carvacrol encapsulation in starch or PCL based matrices by electrospinning. J. Food Eng. 2017, 214, 245–256. [Google Scholar] [CrossRef]
- Galindo-Pérez, M.J.; Quintanar-Guerrero, D.; de los Ángeles Cornejo-Villegas, M.; de la Luz Zambrano-Zaragoza, M. Optimization of the emulsification-diffusion method using ultrasound to prepare nanocapsules of different food-core oils. LWT Food Sci. Technol. 2018, 87, 333–341. [Google Scholar] [CrossRef]
- Rajaei, A.; Hadian, M.; Mohsenifar, A.; Rahmani-Cherati, T.; Tabatabaei, M. A coating based on clove essential oils encapsulated by chitosan-myristic acid nanogel efficiently enhanced the shelf-life of beef cutlets. Food Packag. Shelf Life 2017, 14, 137–145. [Google Scholar] [CrossRef]
- Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Tanner, C.; Cayot, P.; Karbowiak, T.; Debeaufort, F. Impact of functional properties and release kinetics on antioxidant activity of biopolymer active films and coatings. Food Chem. 2018, 242, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Flores-López, M.L.; Cerqueira, M.A.; de Rodríguez, D.J.; Vicente, A.A. Perspectives on Utilization of Edible Coatings and Nano-laminate Coatings for Extension of Postharvest Storage of Fruits and Vegetables. Food Eng. Rev. 2016, 8, 292–305. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Jung, J.; Simonsen, J.; Wang, Y.; Zhao, Y. Cellulose nanocrystal reinforced chitosan coatings for improving the storability of postharvest pears under both ambient and cold storages. J. Food Sci. 2017, 82, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Del Real L., A.; Gutiérrez-Cortez, E.; Cornejo-Villegas, M.A.; Quintanar-Guerrero, D. The effect of nano-coatings with α-tocopherol and xanthan gum on shelf-life and browning index of fresh-cut “red Delicious” apples. Innov. Food Sci. Emerg. Technol. 2014, 22, 188–196. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Amodio, M.L.; Colelli, G. Carvacrol-loaded chitosan nanoparticles maintain quality of fresh-cut carrots. Innov. Food Sci. Emerg. Technol. 2017, 41, 56–63. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, Z.; Zhou, R.; Feng, N. Production of a transparent lavender flavour nanocapsule aqueous solution and pyrolysis characteristics of flavour nanocapsule. J. Food Sci. Technol. 2014, 52, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.E.; Oliveira, D.A.; Hills, K.; Giacobassi, C.; Johnson, J.; Summerlin, H.; Taylor, T.M.; Gomes, C.L. A comparative study of natural antimicrobial delivery systems for microbial safety and quality of fresh-cut lettuce. J. Food Sci. 2017, 82, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Umagiliyage, A.L.; Becerra-Mora, N.; Kohli, P.; Fisher, D.J.; Choudhary, R. Antimicrobial efficacy of liposomes containing D-limonene and its effect on the storage life of blueberries. Postharvest Biol. Technol. 2017, 128, 130–137. [Google Scholar] [CrossRef]
- De León-Zapata, M.A.; Pastrana-Castro, L.; Barbosa-Pereira, L.; Rua-Rodríguez, M.L.; Saucedo, S.; Ventura-Sobrevilla, J.M.; Salinas-Jasso, T.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Nanocoating with extract of tarbush to retard Fuji apples senescence. Postharvest Biol. Technol. 2017, 134, 67–75. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Valle-Marquina, M.Á.; Hernández-López, M. The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on Colletotrichum gloeosporioides Growth in vitro and on cv Hass Avocado and Fruit Quality. J. Phytopathol. 2017, 165, 297–305. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Miranda, K.W.E.; Ribeiro, H.L.; Rosa, M.F.; Nascimento, D.M. Nanoreinforced alginate-acerola puree coatings on acerola fruits. J. Food Eng. 2012, 113, 505–510. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Lira-Moreno, C.Y.; Guerrero-Legarreta, I.; Wild-Padua, G.; Di Pierro, P.; García-Almendárez, B.E.; Regalado-González, C. Effect of nanoemulsified and microencapsulated mexican oregano (Lippia graveolens Kunth) essential oil coatings on quality of fresh pork meat. J. Food Sci. 2017, 82, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A.; Hallaj, R. Influence of nano-ZnO on microbial growth, bioactive content and postharvest quality of strawberries during storage. Innov. Food Sci. Emerg. Technol. 2016, 35, 168–176. [Google Scholar] [CrossRef]
- Erbay, E.A.; Dağtekin, B.B.; Türe, M.; Yeşilsu, A.F.; Torres-Giner, S. Quality improvement of rainbow trout fillets by whey protein isolate coatings containing electrospun poly(ε-caprolactone) nanofibers with Urtica dioica L. extract during storage. LWT Food Sci. Technol. 2017, 78, 340–351. [Google Scholar] [CrossRef]
- Ramos, O.L.; Pereira, R.N.; Martins, A.; Rodrigues, R.; Fuciños, C.; Teixeira, J.A.; Pastrana, L.; Xavier Malcata, F.; Vicente, A.A.; Fuci, C.; et al. Design of whey protein nanostructures for incorporation and release of nutraceutical compounds in food. Crit. Rev. Food Sci. Nutr. 2017, 57, 1377–1393. [Google Scholar] [CrossRef] [PubMed]
- Dan, N. Transport and release in nano-carriers for food applications. J. Food Eng. 2016, 175, 136–144. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanostructured emulsions and nanolaminates for delivery of active ingredients: Improving food safety and functionality. Trends Food Sci. Technol. 2017, 60, 12–22. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, J.H.; Papadokostaki, K.G.; Sanopoulou, M. Higuchi’s equation and beyond: Overview of the formulation and application of a generalized model of drug release from polymeric matrices. Int. J. Pharm. 2012, 437, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Mastromatteo, M.; Barbuzzi, G.; Conte, A.; Nobile, M.A. Del Controlled release of thymol from zein based film. Innov. Food Sci. Emerg. Technol. 2009, 10, 222–227. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Jonaitis, T.S.; Card, J.W. A brief review of the occurrence, use, and safety of food-related nanomaterials. J. Food Sci. 2011, 76, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Bustos C, R.O.; Alberti R, F.V.; Matiacevich, S.B. Edible antimicrobial films based on microencapsulated lemongrass oil. J. Food Sci. Technol. 2016, 53, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Bourbon, A.I.; Quintas, M.A.C.; Coimbra, M.A.; Vicente, A.A. K-Carrageenan/Chitosan Nanolayered Coating for Controlled Release of a Model Bioactive Compound. Innov. Food Sci. Emerg. Technol. 2012, 16, 227–232. [Google Scholar] [CrossRef]
- Eltayeb, M.; Stride, E.; Edirisinghe, M. Preparation, characterization and release kinetics of ethylcellulose nanoparticles encapsulating ethylvanillin as a model functional component. J. Funct. Foods 2015, 14, 726–735. [Google Scholar] [CrossRef]
- Noronha, C.M.; De Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterization of antioxidant methylcellulose film incorporated with α-tocopherol nanocapsules. Food Chem. 2014, 159, 529–535. [Google Scholar] [CrossRef] [PubMed]

| Antioxidant Compound | Nanostructure Functionalized | Findings |
|---|---|---|
| Curcumin | Alginate-pectine/Zein core-shell NPs, (A-P/Z-NPs) | A-P/Z-NPs shown to have superior antioxidant and radical scavenging activities than curcumin solubilized in ethanol [47] |
| Fisetin | PCL-NPs | NPs can be proposed as an attractive delivery system to control the release of antioxidant fisetin for nutraceutical application [48] |
| Gallic acid | Zein ultrafine fibers | Gallic acid had retained its antioxidant activity after incorporation in zein electrospun fibers [49] |
| Quercetin | PLGA-NCs | The developed NCs provide a system for targeted delivery of a range of hydrophobic antioxidant compounds [50] |
| Quercetin and Ferulic acid | Amaranth protein isolated/Pullulan nanofibers | Both bioactives showed a sustained release, keeping a greater extent their antioxidant capacity in comparison with non-functionalized compounds [51] |
| Catechin and Epicatechin | Bovine serum albumin NPs | NPs showed satisfactory sustained release, maintained antioxidant potential and found improved efficacy [52] |
| Catechins from white tea extract | PCL/Alginate NPs | NPs protected tea polyphenols from degradation thus opening new perspectives for the exploitation of white tea extract-loaded NPs for nutraceutical applications [53] |
| Savory essential oil | CS-NPs | Encapsulation enables stronger antioxidant activity to phenolics as compared to their pure forms by entrapping them into capsules and protecting from negative effects of environmental conditions [54] |
| Lippia sidoides essential oil | CG/CS-NGs | In vitro release profiles revealed a prolonged. These results showed that the CG/CS nanogels were designed and present sustained release features [55] |
| Retynil palmitate (Vitamin A) | Palmityol/CS-NPs | The submicron particles can be used as antioxidant systems to improve biodisponibility of vitamins [56] |
| α-Tocopherol (Vitamin E) | Alginate/α-tocopherol NPs | The authors reported an improvement of biodisponibility of α-tocopherol through the encapsulation of the oily antioxidant compound [43] |
| Folic acid (Vitamin B9) | Soy protein/Soy polysaccharide NGs | The protein and polysaccharide can inhibit the reactions between dissolved oxygen and folic acid during UV irradiation. The NGs are a suitable delivery system of folic acid in food and beverages [57] |
| Astaxanthin | Astaxanthin NLC | NLC containing nutraceuticals have potential to be used for functional beverages/food development [58] |
| β-carotene | Cocoa butter SLN | β-carotene degradation was observed during storage. SLN showed an increase in particle size (35%) and color change (ΔE = 20) after 8 days of storage. The authors mentioned that blending other kinds of fats in the SLN’s production will avoid the partial coalescence of lipid crystals and expulsion of carotenoids, leading in physical and chemical stability improved [59] |
| Antimicro-Bial Compound | Nanostructure | Applica-Tion | Targeted and Inhibition Microorganism | Inhibition or Reduction |
|---|---|---|---|---|
| ZnO | ZnO NPs (10–17 nm) | in vitro | E. coli, P. aeruginosa, S. aureus, B. subtilis | 14, 18, 13, 14 mm of zone inhibition/1 cm sample [66] |
| ZnO | ZnO NPs | Chitosan edible coating | E. coli | Total inhibition [67] |
| Nisin and EDTA | NSs (130–270 nm) | in vitro | E. coli, S. aureus | 8 log (CFU/mL) after 24 h 2.3 log (CFU/mL) after 24 h [68] |
| Nisin | SLN (159–167 nm) | in vitro | L. monocytogenes, L. plantarum | 8.5 log (CFU/mL) after 24 h 8 log (CFU/mL) after 24 h [69] |
| Lactoferrin | L-NVs (100–200 nm) | in vitro | S. aureus, Salmonella sp., E. coli, P. fluorescens, L. innocua, B. cereus C. albicans | Minimum inhibitory concentration (MIC): 2000 μg/mL (S. aureus, L. innocua, B. cereus); 200 μg/mL (C. albicans) Not antimicrobial activity observed for Salmonella sp., E. coli, P. fluorescens [70] |
| Eugenol Trans-cinnamaldehyde | PLGA-NPs (174–317 nm) | in vitro | Salmonella spp., Lysteria spp. | MIC: 800 μg/mL 1600 μg/mL [71] |
| Oregano essential oil (Carvacol, Thymol) (6:1) | Liposomes (263–287 nm) | in vitro | S. aureus, S. aeroginosa, E. cloacae, K. pneumoniae, E. coli, S. mutans, S. viridans, C. albicans, C. tropicalis, C. glabrata, L. monocytogenes | 17, 13.3, 12, 13, 14, 17, 17, 12, 14.1, 14.1, 17 mm of zone inhibition [72] |
| Carvacol, p-cymene | Pullulan | Edible coating for Turkey deli meat | S. aureus, L. monocytogenes | 28 and 25.5 mm of zone inhibition after 7 weeks of storage at 25 °C [73] |
| Garlic essential oil | PEG-NPs (<240 nm) | in vitro | T. castaneum | >80% mortality at dose of 8000 mg/kg [74] |
| Cardamom essential oil | CS-NPs (50–100 nm) | in vitro | E. coli S. aureus | CS-NP inhibited the growth of pathogens till first 48 h (amount not mentioned) [75] |
| Peppermint oil | TG-NCs (22 nm) | in vitro | E. coli, S. aureus, C. albicans | Antibacterial/antifungal activities was 100% after 12 h [76] |
| Electrolyzed water-chitosan (EW-C) | Obscure puffer fish rinsed with EW and coated with chitosan | Edible coating | Aerobic bacteria | EW-C treatment retarded the increase in the total viable counts reaching 4.69 log (CFU/g) after 6 days of storage [63] |
| Microorganism | Matrix | Application | Findings |
|---|---|---|---|
| Lactobacillus casei Lactobacillus brevis Lactobacillus plantarum | Resistant starch from rice | Microencapsulation | The viability of (> 7 log CFU/g) for 2 months at 4 °C [86] |
| Lactobacillus acidophilus L. casei, L. rhamnosus, Bifi-dobacterium bifidum | CMC | Edible films | Viability of 107 CFU/g [95] |
| Bifidobacterium animalis Bb12® Lactobacillus casei-01 | Whey protein isolates | Edible coatings on sliced ham preservation | Viability of 108 CFU/g and inhibited detectable growth of Staphylococcus spp., Pseudomonas spp., Enterobacteriaceae [96] |
| Lactobacillus bulgaricus | Whey protein isolate Alginate coating | Microencapsulation | Microencapsulated cells exhibited much better retainability of cell survival during storage, especially under low temperatures [97] |
| Nanofibers of chitin, lignocellulose and bacterial cellulose | Pectin | Nanofibers biocomposites | The optimal biocomposite exhibited the highest survival of the entrapped probiotic bacteria under simulated gastric (97.7%) and intestinal (95.8%) conditions [98] |
| Bacillus subtilis HFC103 | Candelilla wax | Edible coatings in strawberry quality during the shelf life | Effective to control R. stolonifer [99] |
| Lactobacillus plantarum | Pectin Starch | Hydrogel particles by extrusion method | The numbers of surviving cells were 5.15 and 6.67 Log CFU/g for pectin and pectin/starch hydrogel, respectively [100] |
| Bacillus coagulans | Bacterial nanocellulose, Pectin Schizophyllum commune extract | Bionanocomposites | Survivability of probiotic under drying process and gastrointestinal condition. During storage period at ambient temperature, 4 °C and −20 °C performed viability reduction: 1.3, 1.7 and 1.8 log CFU/g [101] |
| Lactobacillus plantarum, | Whey protein | Electrospraying conditions for the microencapsula-tion | Viability losses lower than 1 log10 CFU and the bacterial counts of the final products exceeded 8.5 log10 CFU/g [102] |
| Lactobacillus casei | Inulin incorporated into alginate and chitosan coated alginate beads | Microencapsula-tion | Using inulin and chitosan-coating, the survival of co-encapsulated cells in simulated gastro-intestinal condition was improved with only 2.7–2.9 log [103] |
| Fructooligosaccharides | Cassava starch | Edible films | The addition of FOSs resulted in higher solubility and elongation, a decreased water vapor permeability of the films [104] |
| Bifidobacterium animalis Bb12® Lactobacillus casei-01 | Whey protein isolate | Edible films | Viability of 106 CFU/g film until Day 60 at 23 and 4 °C [105] |
| Lactobacillus rhamnosus GG | Native rice, corn starch, bovine skin gelatin, sodium caseinate and soy protein concentrate | Edible films | Loss of L. rhamnosus in presence of proteins (0.91–1.07 log CFU/g) and starch based systems (1.71 log CFU/g) [106] |
| Fructooligosaccharides Lactobacillus plantarum CIDCA 83114 | Methylcellulose | Green apple baked snacks functionalized with edible coatings | Bacteria were still alive after the simulated gastric (5.5 × 106 ± 0.7 × 106 CFU/g) and intestinal (1.0 × 106 ± 0.4 × 106 CFU/g) [107] |
| Lactobacillus plantarum CIDCA Kluyveromyces marxianus | Kefiran | Edible film | L. plantarum viability decreased less than 1.3 logarithmic cycles and K. marxianus 0.7 logarithmic cycles [108] |
| Lactobacillus rhamnosus GG Pre-biotic: inulin, polydextrose, glucose-oligosaccharides and wheat dextrin | Gelatine | Edible films | The supplementation of edible films helps to maintain the viability of L. rhamnosus GG and ameliorated the storage microbiological stability of L. rhamnosus GG at different temperatures [109] |
| Lactobacillus rhamnosus GG | Sodium alginate-WPC | Edible films for bread | A lower viability percentage was determined in the case of the breads coated with sodium alginate (15%) compared to those containing WPC (76.3%) [110] |
| Lactobacillus delbrueckii subsp. bulgaricus CIDCA333 Lactobacillus plantarum CIDCA 83114 | Methylcellulose and fructo-oligosaccharides | Edible films | Viability of 1.1 × 1010 ± 1.6 × 109 CFU/mL for L. delbrueckii subsp. bulgaricus and 2.7 × 1012 ± 8.7 × 1011 CFU/mL for L. plantarum [111] |
| Colorant | Matrix | Findings |
|---|---|---|
| β-carotene | PCL | NCs provided a little aggregation between NPs of β-carotene during storage at 25 °C for 28 days [116] |
| Anthocyanins from purple sweet potato | Konjac glucomannan/Chitosan | NCs of anthocyanins revealed that the compounds were stable up to pH 3 with controlled release of the active ingredient [117] |
| Anthocyanins from red cabbage | Palmitic acid | The SLN containing anthocyanins was successfully achieved, showing high entrapment efficiency (>90%), decreasing degradation of these compounds against pH and temperature [118] |
| Lycopene | PCL | NPs provides a protecting effect that impedes the quick degradation of lycopene under light, oxygen and temperature, which makes it a potential colorant for edible coatings [119] |
| β-carotene | PCL/Xanthan gum | Nanocoatings with carotene/PCL NCs in a xanthan gum matrix help preserve the color of fresh cut melon for 21 days at 4 °C [120] |
| Anthocyanins from black carrot | WPC | Formation of nanogels of anthocyanins have greater physical stability (the composite gel microcapsules could protect the color) in dairy products [121] |
| Tea polyphenols/β-carotene | CMC-CS/Zein | The powders obtained have a high dissolution rate and better solubility properties. The percentage of rehydration was greater than 90% [122] |
| Anthocyanins from black carrot | Gelatin/CS | It was found that the release of the anthocyanins was a function of the concentration of chitosan, the encapsulation efficiency was relatively high (greater in all cases than 75%) [123] |
| Flavor | Matrix | Food | Findings |
|---|---|---|---|
| Cinnamon and oregano | BCD/CS | in vitro | The encapsulation favored the retention of volatile compounds in the formation of nanofibers [125] |
| Carvacrol | Starch/PCL | in vitro | Nanostructure presented better cohesiveness and adhesiveness in the electrospun material, which also contribute to the coating performance [126] |
| Curcumin | Gelatin | Gellified fish product | Curcumin encapsulation increase water solubility and improving its dispersion/solubility in the aqueous food matrix used as a food model [126] |
| Cardamom | Cocoa butter | in vitro | NLC had fine size (<150 nm) and high entrapment efficiency (>90%) [26] |
| Rosemary | PCL or Ethyl cellulose | in vitro | NCs prepared by ultrasound had sizes smaller than 200 nm with high entrapment efficiency (>70%) with applications in food conservation [127] |
| Clove | CS | Beef cutlets | The application of nanogels was effective to preserve the natural color of the product, in addition to preventing the volatility and instability of the active substance [128] |
| Black pepper | BCD | in vitro | Nanostructures with BCD can maintain the bioactive properties of the essential oil of black pepper [115] |
| Vanillin | BCD | in vitro | Inclusion nanostructures of vanillin, enhancing its antioxidant capacity, improving its functionality as a flavoring agent [129] |
| Bioactive Substance | Nanostructure/Wall Material | Matrix of Edible Coating | Ingredient Type | Food | Findings |
|---|---|---|---|---|---|
| Cinnamon bark extract (CBE) | NCs: PLGA/Poly-N-isopropyl acril amide (PNIPAAM) | CS | Natural, plant derived compounds | Fresh-cut lettuce | NCs with PLGA were prepared by emulsion-evaporation method. NCs were most efficient for Antimicrobial effect for L. monocytogenes, that those prepared with PNIPAAM [138] |
| D-limonene | Liposomes | CS | Others (pure ingredient) | Blueberries | The limonene encapsulated in liposomes had good functionality and inhibit the growth of Botrytis cinirea, E. coli and L. monocytogenes. Moreover, kept the quality for nine weeks of storage at 4 °C [139] |
| Tarbush (phyto molecules) | Candelilla wax/CS-NPs | Arabic gum | Natural, plant derived compounds | Apples | Nanocoatings were prepared by hot homogenization method and applied on surface of apples- shown that use of Phyto molecules reduce the color changes and the action of polyphenol oxidases increase the shelf life by 8 weeks [140] |
| Thyme oil | CS-NPS | Canola oil/glycerol | Natural extract | Avocado | Thyme oil concentration had beneficial effect over the shelf life of avocado. The high concentration was more effective for inhibition of C. gloesporiodies [141] |
| Acerola puree | Nanocomposites Montmorillonite (MMT), Cellulose whiskers (CWAA) | alginate/acelora puree | By-product vegetal origin | Acerola | MMT and CWAA were used as enforcement in edible coating of acerola puree and employed in acerola fruit preservation contribute to the film forming dispersions, improved the ascorbic acid retention [142] |
| Mexican oregano essential oil | NCs Modified starch | Modified Starch | Natural extract | Pork meat | Inhibition of microbial growth on meat previously inoculated with Brochothrix thermosphacta, Micrococcus luteus, Lactobacillus plantarum, Pseudomonas fragi, and Salmonella Infantis was tested [143] |
| Nano-ZnO | Nanoreinforced | CMC | Inorganic compounds | Pomegranate | Edible coatings based on 0.5% of CMC and 0.2% of nano-ZnO, contributed to maintaining the quality parameters of pomegranate [144] |
| Extract of Urtica dioica L. | Nanofibers PCL | WPI | Natural ingredient Extrac plant and animal protein | Rainbow trout | Coatings were applied on rainbow trout fillets, showing that nanofibers inhibit the growth of bacteria’s (lactic acid and mesophilic) and reduce the total volatile basic nitrogen and thiobarbituric acid [145] |
| Active Component | Nanostructure Type | Model of Release | Release Type |
|---|---|---|---|
| Limon essential oil | NCs: Sodium caseinate | Exponential, Higuchi, and Weilbull Model | Correlations between films with microcapsules of lemongrass essential oil and this oil without microcapsules in films of alginate matrix. Peppas model, showed values of the η exponent in both cases was in the zone indicative of a Fickian release mechanism: n = 0.205 < 0.43 for microcapsule spheres and n = 0.311 < 0.5 for thin films [157] |
| Cooper cations | Microcapsules: Alginate | Kinetic model | The release constant k increases with initial copper cation concentration. Relatively high η values (n > 0.43) indicate copper cation release following anomalous kinetics (diffusion and polymer relaxation). T. viride released in the surrounding solution [158] |
| Ethylvanillin | NPs: Ethylcellulose | Ritger–Peppas and first order release model | The values of η for all concentrations of polymers in the NPs prepared were found to be >0.48 and <0.89, indicating that release occurred through a non-Fickian diffusion mechanism [159] |
| α-tocopherol | NCs: Methylcellulose | Fickian release | The release profiles of all NCs films exhibited an initial burst effect (first hour), followed by a sustained release over 10 days, with a typical Fick’s curve [160] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Reza, R.M.; García-Betanzos, C.I.; Sánchez-Valdes, L.I.; Quintanar-Guerrero, D.; Cornejo-Villegas, M.A.; Zambrano-Zaragoza, M.L. The Functionalization of Nanostructures and Their Potential Applications in Edible Coatings. Coatings 2018, 8, 160. https://doi.org/10.3390/coatings8050160
González-Reza RM, García-Betanzos CI, Sánchez-Valdes LI, Quintanar-Guerrero D, Cornejo-Villegas MA, Zambrano-Zaragoza ML. The Functionalization of Nanostructures and Their Potential Applications in Edible Coatings. Coatings. 2018; 8(5):160. https://doi.org/10.3390/coatings8050160
Chicago/Turabian StyleGonzález-Reza, Ricardo M., Claudia I. García-Betanzos, Liliana I. Sánchez-Valdes, David Quintanar-Guerrero, María A. Cornejo-Villegas, and María L. Zambrano-Zaragoza. 2018. "The Functionalization of Nanostructures and Their Potential Applications in Edible Coatings" Coatings 8, no. 5: 160. https://doi.org/10.3390/coatings8050160
APA StyleGonzález-Reza, R. M., García-Betanzos, C. I., Sánchez-Valdes, L. I., Quintanar-Guerrero, D., Cornejo-Villegas, M. A., & Zambrano-Zaragoza, M. L. (2018). The Functionalization of Nanostructures and Their Potential Applications in Edible Coatings. Coatings, 8(5), 160. https://doi.org/10.3390/coatings8050160