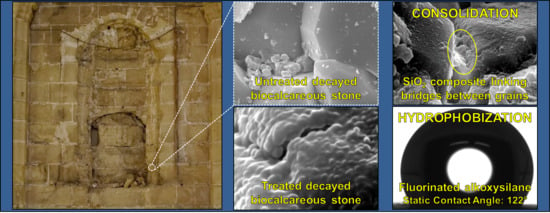
New Consolidant-Hydrophobic Treatment by Combining SiO2 Composite and Fluorinated Alkoxysilane: Application on Decayed Biocalcareous Stone from an 18th Century Cathedral
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Monumental Building Stone
2.2. Consolidant and Hydrophobic Products and Application Procedure
2.3. Effectiveness Evaluation on Biocalcareous Stone
2.3.1. Effectiveness on Quarry Stone
2.3.2. Effectiveness on Monumental Stone Extracted from Jerez de la Frontera Cathedral
3. Results and Discussion
3.1. Evaluation of the Effectiveness on Quarry Stone
3.2. Effectiveness on Monumental Stone Extracted from Jerez de la Frontera Cathedral
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poli, T.; Toniolo, L.; Chiantore, O. The protection of different Italian marbles with two partially flourinated acrylic copolymers. Appl. Phys. A Mater. Sci. Process. 2004, 79, 347–351. [Google Scholar] [CrossRef]
- Graedel, T.E.; McGill, R. Degradation of materials in the atmosphere. Environ. Sci. Technol. 1986, 20, 1093–1100. [Google Scholar] [CrossRef]
- Dolske, D.A. Deposition of atmospheric pollutants to monuments, statues, and buildings. Sci. Total Environ. 1995, 167, 15–31. [Google Scholar] [CrossRef]
- Villegas-Sánchez, R.; Arroyo, F. The cathedral of Jerez De La Frontera (Cádiz, Spain): Stone degradation and conservation. J. Cult. Herit. 2013, 14, e113–e116. [Google Scholar] [CrossRef]
- Wheeler, G. Alkoxysilanes and the Consolidation of Stone; The Getty Conservation Institute: Los Angeles, CA, USA, 2005. [Google Scholar]
- Scherer, G.W.; Wheeler, G.S. Silicate consolidants for stone. Key Eng. Mater. 2009, 391, 1–25. [Google Scholar] [CrossRef]
- Mosquera, M.J.; de los Santos, D.M.; Montes, A.; Valdez-Castro, L. New nanomaterials for consolidating stone. Langmuir 2008, 24, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, M.J.; Desireé, M.; Valdez-Castro, L.; Esquivias, L. New route for producing crack-free xerogels: Obtaining uniform pore size. J. Non-Cryst. Solids 2008, 354, 645–650. [Google Scholar] [CrossRef]
- Mosquera, M.J.; de los Santos, D.M.; Rivas, T.; Sanmartín, P.; Silva, B. New nanomaterials for protecting and consolidating stone. J. Nano Res. 2009, 8, 1–12. [Google Scholar] [CrossRef]
- Mosquera, M.J.; de los Santos, D.M.; Rivas, T. Surfactant-synthesized ormosils with application to stone restoration. Langmuir 2010, 26, 6737–6745. [Google Scholar] [CrossRef] [PubMed]
- Illescas, J.F.; Mosquera, M.J. Surfactant-synthesized PDMS/silica nanomaterials improve robustness and stain resistance of carbonate stone. J. Phys. Chem. C 2011, 115, 14624–14634. [Google Scholar] [CrossRef]
- Illescas, J.F.; Mosquera, M.J. Producing surfactant synthesized nanomaterials in situ on a building susbstrate, without volatile organic compounds. ACS Appl. Mater. Interfaces 2012, 4, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Facio, D.S.; Mosquera, M.J. Simple strategy for producing superhydrophobic nanocomposite coatings in situ on a building substrate. ACS Appl. Mater. Interfaces 2013, 5, 7517–7526. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Mosquera, M.J. Titania-silica nanocomposite photocatalysts with application in stone self-cleaning. J. Phys. Chem. C 2011, 115, 22851–22862. [Google Scholar] [CrossRef]
- Pinho, L.; Mosquera, M.J. Photocatalytic activity of TiO2-SiO2 nanocomposites applied to buildings: Influence of particle size and loading. Appl. Catal. B Environ. 2013, 134–135, 205–221. [Google Scholar] [CrossRef]
- Pinho, L.; Rojas, M.; Mosquera, M.J. Ag–SiO2–TiO2 nanocomposite coatings with enhanced photoactivity for self-cleaning application on building materials. Appl. Catal. B Environ. 2015, 178, 144–154. [Google Scholar] [CrossRef]
- De Rosario, I.; Elhaddad, F.; Pan, A.; Benavides, R.; Rivas, T.; Mosquera, M.J. Effectiveness of a novel consolidant on granite: Laboratory and in situ results. Constr. Build. Mater. 2015, 76, 140–149. [Google Scholar] [CrossRef]
- De Rosario, I.; Rivas, T.; Buceta, G.; Feijoo, J.; Mosquera, M.J. Surfactant-synthesized consolidants applied to a granitic medieval necropolis in NW Spain. Laboratory and in situ effectiveness evaluation. Int. J. Archit. Herit. 2017, 11, 1166–1176. [Google Scholar] [CrossRef]
- Facio, D.S.; Luna, M.; Mosquera, M.J. Facile preparation of mesoporous silica monoliths by an inverse micelle mechanism. Microporous Mesoporous Mater. 2017, 247, 166–176. [Google Scholar] [CrossRef]
- Scherer, G. Theory of drying. J. Am. Ceram. Soc. 1990, 73, 3–14. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Li, D.; Xiang, N.; Zhang, Q.; Shao, L. Solvent effects on structural properties of SiO2 gel using n-octylamine as a catalyst. J. Sol-Gel Sci. Technol. 2014, 71, 204–210. [Google Scholar] [CrossRef]
- Xu, F.; Li, D.; Zhang, Q.; Zhang, H.; Xu, J. Effects of addition of colloidal silica particles on TEOS-based stone protection using n-octylamine as a catalyst. Prog. Org. Coat. 2012, 75, 429–434. [Google Scholar] [CrossRef]
- Simionescu, B.; Olaru, M.; Aflori, M.; Cotofana, C. Silsesquioxane-based hybrid nanocomposite with self-assembling properties for porous limestones conservation. High Perform. Polym. 2010, 22, 42–55. [Google Scholar] [CrossRef]
- Simionescu, B.; Aflori, M.; Olaru, M. Protective coatings based on silsesquioxane nanocomposite films for building limestones. Constr. Build. Mater. 2009, 23, 3426–3430. [Google Scholar] [CrossRef]
- Mosquera, M.J.; Bejarano, M.; de la Rosa-Fox, N.; Esquivias, L. Producing crack-free colloid—Polymer hybrid gels by tailoring porosity. Langmuir 2003, 19, 951–957. [Google Scholar] [CrossRef]
- Miliani, C.; Velo-Simpson, M.L.; Scherer, G.W. Particle-modified consolidants: A study on the effect of particles on sol-gel properties and consolidation effectiveness. J. Cult. Herit. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Salazar-Hernández, C.; Alquiza, M.J.P.; Salgado, P.; Cervantes, J. TEOS-colloidal silica-PDMS-OH hybrid formulation used for stone consolidation. Appl. Organomet. Chem. 2010, 24, 481–488. [Google Scholar] [CrossRef]
- Liu, R.; Han, X.; Huang, X.; Li, W.; Luo, H. Preparation of three-component TEOS-based composites for stone conservation by sol-gel process. J. Sol-Gel Sci. Technol. 2013, 68, 19–30. [Google Scholar] [CrossRef]
- Pinho, L.; Elhaddad, F.; Facio, D.S.; Mosquera, M.J. A novel TiO2-SiO2 nanocomposite converts a very friable stone into a self-cleaning building material. Appl. Surf. Sci. 2013, 275, 389–396. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Pavlou, A.; Manoudis, P.N.; Aifantis, K.E. Aifantis, Water repellent ORMOSIL films for the protection of stone and other materials. Mater. Lett. 2014, 131, 276–279. [Google Scholar] [CrossRef]
- Kronlund, D.; Bergbreiter, A.; Meierjohann, A.; Kronberg, L.; Lindén, M.; Grosso, D.; Smått, J.H. Hydrophobization of marble pore surfaces using a total immersion treatment method—Product selection and optimization of concentration and treatment time. Prog. Org. Coat. 2015, 85, 159–167. [Google Scholar] [CrossRef]
- Colangiuli, D.; Calia, A.; Bianco, N. Novel multifunctional coatings with photocatalytic and hydrophobic properties for the preservation of the stone building heritage. Constr. Build. Mater. 2015, 93, 189–196. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Superhydrophobic composite films produced on various substrates. Langmuir 2008, 24, 11225–11232. [Google Scholar] [CrossRef] [PubMed]
- Facio, D.S.; Carrascosa, L.A.; Mosquera, M.J. Producing lasting amphiphobic building surfaces with self-cleaning properties. Nanotechnology 2017, 28, 265601. [Google Scholar] [CrossRef] [PubMed]
- Rescic, S.; Fratini, F.; Tiano, P. On-site evaluation of the “mechanical” properties of Maastricht limestone and their relationship with the physical characteristics. Geol. Soc. Lond. Spec. Publ. 2010, 331, 203–208. [Google Scholar] [CrossRef]
- UNE-EN 1925:1999 Natural Stone Test Methods. Determination of Water Absorption Coefficient by Capillarity; AENOR: Madrid, Spain, 1999.
- Mosquera, M.J.; Benı́tez, D.; Perry, S.H. Pore structure in mortars applied on restoration. Cem. Concr. Res. 2002, 32, 1883–1888. [Google Scholar] [CrossRef]
- ASTM E 96-90 Standard Test Methods for Water Vapor Transmission of Materials; ASTM: West Conshohocken, PA, USA, 1990.
- Berns, R.S. Billmeyer and Saltzman’s Principles of Color Technology; Wiley-Interscience: New York, NY, USA, 2000. [Google Scholar]
- Ling, X.Y.; Phang, I.Y.; Vancso, G.J.; Huskens, J.; Reinhoudt, D.N. Stable and transparent superhydrophobic nanoparticle films. Langmuir 2009, 25, 3260–3263. [Google Scholar] [CrossRef] [PubMed]
- UNE-EN ISO 2409:2013 Paints and Varnishes. Cross-Cut Test; AENOR: Madrid, Spain, 2013.
- Carrascosa, L.A.; Facio, D.S.; Mosquera, M.J. Producing superhydrophobic roof tiles. Nanotechnology 2016, 27, 095604. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Oguchi, C.T. Role of pore size distribution in salt uptake, damage, and predicting salt susceptibility of eight types of Japanese building stones. Eng. Geol. 2010, 115, 226–236. [Google Scholar] [CrossRef]
- Noble, K.; Seddon, A.B.; Turner, M.L.; Chevalier, P.; Mackinnon, I.A. Polysiloxane-modified mesoporous materials. J. Sol-Gel Sci. Technol. 2000, 19, 807–810. [Google Scholar] [CrossRef]
- Della Volpe, C.; Penati, A.; Peruzzi, R.; Siboni, S.; Toniolo, L.; Colombo, C. The combined effect of roughness and heterogeneity on contact angles: the case of polymer coating for stone protection. J. Adhes. Sci. Technol. 2000, 14, 273–299. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Large contact angles of plants and animal surfaces. Nature 1945, 155, 21–22. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Pedna, A.; Pinho, L.; Frediani, P.; Mosquera, M.J. Obtaining SiO2-fluorinated PLA bionanocomposite with application as reversible and highly-hydrophobic coatings of buildings. Prog. Org. Coat. 2015, 90, 91–100. [Google Scholar] [CrossRef]
- Bhushan, B.; Her, E.K. Fabrication of superhydrophobic surfaces with high and low adhesion inspired from rose petal. Langmuir 2010, 26, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Elhaddad, F.; Carrascosa, L.A.M.; Mosquera, M.J. Long-term effectiveness, under coastal environment, of a novel conservation nanomaterial applied on sandstone from Roman archaeological site. J. Cult. Herit. 2018, in press. [Google Scholar]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]









| Treatment | Uptake (%) | Dry Matter (%) | DM/U (%) |
|---|---|---|---|
| TV100 | 5.70 ± 2.93 | 3.17 ± 1.43 | 56 |
| BS290 | 4.41 ± 2.24 | 0.58 ± 0.24 | 13 |
| UCA-T | 5.63 ± 1.19 | 4.11 ± 0.63 | 73 |
| UCA-TS | 5.65 ± 0.72 | 4.46 ± 0.58 | 79 |
| UCA-T+F | 5.59 ± 0.60 | 3.97 ± 0.33 | 71 |
| UCA-TS+F | 5.71 ± 0.17 | 4.10 ± 0.17 | 72 |
| Treatment | D·10−6 (m2·s−1) | DR (%) | TWU (%) | ΔE* (1 Month) | ΔE* (1 Year) |
|---|---|---|---|---|---|
| Untreated | −10.04 ± 2.12 | – | 15.31 ± 1.29 | – | – |
| TV100 | −7.03 ± 0.22 | 30 | 4.02 ± 0.01 | 2.12 ± 0.69 | 2.23 ± 1.33 |
| BS290 | −8.53 ± 0.36 | 15 | 0.48 ± 0.13 | 10.12 ± 2.11 | 6.03 ± 0.69 |
| UCA-T | −6.95 ± 0.80 | 31 | 0.35 ± 0.10 | 10.59 ± 1.71 | 6.12 ± 1.69 |
| UCA-TS | −6.21 ± 0.13 | 38 | 0.33 ± 0.00 | 11.16 ± 3.06 | 5.97 ± 2.27 |
| UCA-T + F | −6.96 ± 1.12 | 31 | 0.22 ± 0.10 | 8.72 ± 0.81 | 4.76 ± 0.89 |
| UCA-TS + F | −6.02 ± 0.04 | 40 | 0.20 ± 0.04 | 9.12 ± 0.78 | 4.91 ± 1.75 |
| Treatment | Uptake (%) | Dry Matter (%) | ΔE* | Adhesion Test (mg) |
|---|---|---|---|---|
| Untreated | – | – | – | 9.3 ± 9.4 |
| UCA-TS + F | 4.83 ± 0.52 | 3.64 ± 0.40 | 10.65 ± 0.99 | 0.1 ± 0.2 |
| Treatment | θS (°) | θA (°) | θR (°) | Hysteresis (°) | TWU (%) |
|---|---|---|---|---|---|
| Untreated | – | – | – | – | 12.50 |
| UCA-TS+F | 123 ± 2 | 118 ± 1.13 | 101 ± 3 | 17.91 ± 1.97 | 0.19 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facio, D.S.; Ordoñez, J.A.; Gil, M.L.A.; Carrascosa, L.A.M.; Mosquera, M.J. New Consolidant-Hydrophobic Treatment by Combining SiO2 Composite and Fluorinated Alkoxysilane: Application on Decayed Biocalcareous Stone from an 18th Century Cathedral. Coatings 2018, 8, 170. https://doi.org/10.3390/coatings8050170
Facio DS, Ordoñez JA, Gil MLA, Carrascosa LAM, Mosquera MJ. New Consolidant-Hydrophobic Treatment by Combining SiO2 Composite and Fluorinated Alkoxysilane: Application on Decayed Biocalcareous Stone from an 18th Century Cathedral. Coatings. 2018; 8(5):170. https://doi.org/10.3390/coatings8050170
Chicago/Turabian StyleFacio, Dario S., Jose A. Ordoñez, M. L. Almoraima Gil, Luis A. M. Carrascosa, and Maria J. Mosquera. 2018. "New Consolidant-Hydrophobic Treatment by Combining SiO2 Composite and Fluorinated Alkoxysilane: Application on Decayed Biocalcareous Stone from an 18th Century Cathedral" Coatings 8, no. 5: 170. https://doi.org/10.3390/coatings8050170
APA StyleFacio, D. S., Ordoñez, J. A., Gil, M. L. A., Carrascosa, L. A. M., & Mosquera, M. J. (2018). New Consolidant-Hydrophobic Treatment by Combining SiO2 Composite and Fluorinated Alkoxysilane: Application on Decayed Biocalcareous Stone from an 18th Century Cathedral. Coatings, 8(5), 170. https://doi.org/10.3390/coatings8050170