Relationship Processing–Composition–Structure–Resistivity of LaNiO3 Thin Films Grown by Chemical Vapor Deposition Methods
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Evaporation and Decomposition of La and Ni Precursors
3.1.1. Effect of Solvent
3.1.2. Evaporation Temperature
3.1.3. Deposition Pressure and Injection Frequency
3.1.4. Deposition Temperature
3.1.5. La/Ni Ratio in Solution
3.2. Properties of LaNiO3 Thin Films
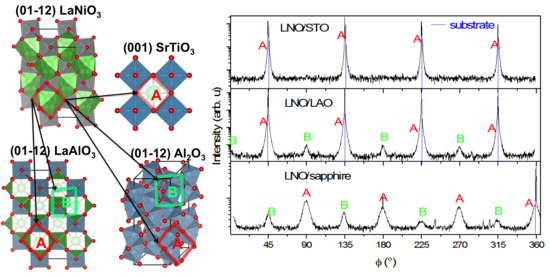
3.2.1. Structural Properties
3.2.2. Resistivity
4. Discussion
- (110)LAO‖(110)LNO (−1.6%) and (014)′LAO‖(014)′LNO (−0.5%);
- (110)LAO‖(20)LNO (−1.6%) and (014)′LAO‖(01)′LNO (−0.5%);
- (110)LAO‖(014)′LNO (−0.4%) and (014)′LAO‖(110)LNO (−1.8%);
- (110)LAO‖(01)′LNO (−0.4%) and (014)′LAO‖(20)LNO (−1.8%).
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramesh, R.; Gilchrist, H.; Sands, T.; Keramidas, V.G.; Haakenaasen, R.; Fork, D.K. Ferroelectric La–Sr–Co–O/Pb–Zr–Ti–O/La–Sr–Co–O heterostructures on silicon via template growth. Appl. Phys. Lett. 1993, 63, 3592–3594. [Google Scholar] [CrossRef]
- Satyalakshmi, K.M.; Mallya, R.M.; Ramanathan, K.V.; Wu, X.D.; Brainard, B.; Gautier, D.C.; Vasanthacharya, N.Y.; Hegde, M.S. Epitaxial metallic LaNiO3 thin films grown by pulsed laser deposition. Appl. Phys. Lett. 1993, 62, 1233–1235. [Google Scholar] [CrossRef]
- Aytug, T.; Kang, B.W.; Cantoni, C.; Specht, E.D.; Paranthaman, M.; Goyal, A.; Christen, D.K.; Verebelyi, D.T.; Wu, J.Z.; Ericson, R.E.; et al. Growth and characterization of conductive SrRuO3 and LaNiO3 multilayers on textured Ni tapes for high-Jc YBa2Cu3O7−δ coated conductors. J. Mater. Res. 2001, 16, 2661–2669. [Google Scholar] [CrossRef]
- Garcia-Munoz, J.L.; Rodriguez-Carvajal, J.; Lacorre, P.; Torrance, J.B. Neutron-diffraction study of RNiO3 (R = La, Pr, Nd, Sm): Electronically induced structural changes across the metal-insulator transition. Phys. Rev. B Condens. Matter. 1992, 46, 4414–4425. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Sun, J.; Yu, J.; Wang, G.; Guo, S.; Chu, J. Enhanced fatigue property of PZT thin films using LaNiO3 thin layer as bottom electrode. Appl. Phys. A 2001, 73, 323–325. [Google Scholar] [CrossRef]
- Guerrero, C.; Sanchez, F.; Ferrater, C.; Roldán, J.; García-Cuenca, M.V.; Varela, M. Pulsed laser deposition of epitaxial PbZrxTi1−xO3 ferroelectric capacitors with LaNiO3 and SrRuO3 electrodes. Appl. Surf. Sci. 2000, 168, 219–222. [Google Scholar] [CrossRef]
- Benckiser, E.; Haverkort, M.W.; Brück, S.; Goering, E.; Macke, S.; Frañó, A.; Yang, X.; Andersen, O.K.; Cristiani, G.; Habermeier, H.-U.; et al. Orbital reflectometry of oxide heterostructures. Nat. Mater. 2011, 10, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Jang, A.N.; Seung, S.K.; Choi, K.H.; Song, J.H. Epitaxial growth and electrical transport property of artificial LaNiO3/LaAlO3 superlattices. Ceram. Int. 2012, 38, S627–S630. [Google Scholar] [CrossRef]
- Guo, X.; Li, C.; Zhou, Y.; Chen, Z. High-quality LaNiO3 thin-film electrode grown by pulsed laser deposition. J. Vac. Sci. Technol. A 1999, 17, 917–920. [Google Scholar] [CrossRef]
- Kim, S.S.; Kang, T.S.; Je, J.H. Microstructures of LaNiO3 films grown on Si(001) by pulsed laser deposition. Thin Solid Films 2002, 405, 117–121. [Google Scholar] [CrossRef]
- Li, A.; Ge, C.; Lü, P.; Ming, N. Preparation of perovskite conductive LaNiO3 films by metalorganic decomposition. Appl. Phys. Lett. 1996, 68, 1347–1349. [Google Scholar] [CrossRef]
- Wakiya, N.; Azuma, T.; Shinozaki, K.; Mizutani, N. Low-temperature epitaxial growth of conductive LaNiO3 thin films by RF magnetron sputtering. Thin Solid Films 2002, 410, 114–120. [Google Scholar] [CrossRef]
- Lane, P.A.; Crosbie, M.J.; Wright, P.J.; Donohue, P.P.; Hirst, P.J.; Reeves, C.L.; Anthony, C.J.; Jones, J.C.; Todd, M.A.; Williams, D.J. The metal–organic chemical vapor deposition of lanthanum nickelate electrodes for use in ferroelectric devices. Chem. Vapor Depos. 2003, 9, 87–92. [Google Scholar] [CrossRef]
- Korsakov, I.; Mankevich, A.; Tsymbarenko, D.; Makarevich, A.M.; Murzina, T.; Kaul, A. Epitaxial heterostructures of KNbO3/LaNiO3/SrTiO3 and KNbO3/La1−xKxMnO3/SrTiO3: MOCVD preparation and properties. ECS Trans. 2009, 25, 513–519. [Google Scholar]
- Seim, H.; Mölsä, H.; Nieminen, M.; Fjellvåg, H.; Niinistö, L. Deposition of LaNiO3 thin films in an atomic layer epitaxy reactor. J. Mater. Chem. 1997, 7, 449–454. [Google Scholar] [CrossRef]
- Qiao, L.; Bi, X. Direct observation of Ni3+ and Ni2+ in correlated LaNiO3−δ films. Europhys. Lett. 2011, 93, 57002. [Google Scholar] [CrossRef]
- Kuzmina, N.; Malkerova, I.; Ryazanov, M.; Alikhanyan, A.; Rogachev, A.; Gleizes, A.N. Volatility studies on single source precursors for LaNiO3 film deposition: Mass spectrometry and thermal analysis. J. Phys. IV France 2001, 11, 661–667. [Google Scholar] [CrossRef]
- Hassan, A.J. Study of optical and electrical properties of nickel oxide (NiO) thin films deposited by using a spray pyrolysis technique. J. Mod. Phys. 2014, 5, 2184–2191. [Google Scholar] [CrossRef]
- Gorbenko, O.Y.; Bosak, A.A. Growth of LaNiO3 thin films on MgO by flash MOCVD. J. Cryst. Growth 1998, 186, 181–188. [Google Scholar] [CrossRef]
- Abrutis, A.; Bartasyte, A.; Garcia, G.; Teiserskis, A.; Kubilius, V.; Saltyte, Z.; Faucheux, V.; Figueras, A.; Rushworth, S. Metal-organic chemical vapour deposition of mixed-conducting perovskite oxide layers on monocrystalline and porous ceramic substrates. Thin Solid Films 2004, 449, 94–99. [Google Scholar] [CrossRef]
- Eisentraut, K.J.; Sievers, R.E. Thermogravimetric studies of metal β-diketonates. J. Inorg. Nucl. Chem. 1967, 29, 1931–1936. [Google Scholar] [CrossRef]
- Burriel López, M. Epitaxial Thin Films of Lanthanum Nickel Oxides: Deposition by PI-MOCVD, Structural Characterization and High Temperature Transport Properties. Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, March 2007. [Google Scholar]
- Bartasyte, A.; Bouregba, R.; Dogheche, E.; Boudard, M.; Poullain, G.; Chaix-Pluchery, O.; Jimenez, C.; Plausinaitiene, V.; Remiens, D.; Abrutis, A.; et al. Ferroelectric PbTiO3 films grown by pulsed liquid injection MOCVD. Surf. Coat. Technol. 2007, 201, 9340–9344. [Google Scholar] [CrossRef]
- Bartasyte, A.; Abrutis, A.; Jimenez, C.; Weiss, F.; Chaix-Pluchery, O.; Saltyte, Z. Ferroelectric PbTiO3 films grown by pulsed liquid injection metalorganic chemical vapour deposition. Ferroelectrics 2007, 353, 104–115. [Google Scholar] [CrossRef]
- Kuprenaite, S.; Murauskas, T.; Abrutis, A.; Kubilius, V.; Saltyte, Z.; Plausinaitiene, V. Properties of In-, Ga-, and Al-doped ZnO films grown by aerosol-assisted MOCVD: Influence of deposition temperature, doping level and annealing. Surf. Coat. Technol. 2015, 271, 156–164. [Google Scholar] [CrossRef]
- Cabello, M.; Varghese, A.; Montserrat, J.; Rebollo, J.; Decams, J.M.; Godignon, P. Analysis of ZrxSiyOz as High-k Dielectric for 4H-SiC MOSFETs. Mater. Sci. Forum 2018, 924, 939–942. [Google Scholar] [CrossRef]
- What does DLI mean? Available online: https://www.annealsys.com/applications/dli-processes/what-does-dli-mean-ty20804.html (accessed on 20 September 2018).
- Bartasyte, A.; Plausinaitiene, V.; Abrutis, A.; Stanionyte, S.; Margueron, S.; Kubilius, V.; Boulet, P.; Huband, S.; Thomas, P.A. Thickness dependent stresses and thermal expansion of epitaxial LiNbO3 thin films on C-sapphire. Mater. Chem. Phys. 2015, 149–150, 622–631. [Google Scholar] [CrossRef]
- Astié, V.; Millon, C.; Decams, J.-M.; Bartasyte, A. Direct liquid injection chemical vapor deposition. In Chemical Vapor Deposition for Nanotechnology; Pietro, M., Ed.; InTech: Vienna, Austria, 2018. [Google Scholar]
- Soukup, R.W.; Schmid, R. Metal complexes as color indicators for solvent parameters. J. Chem. Educ. 1985, 62, 459. [Google Scholar] [CrossRef]
- Jones, A.C. MOCVD of electroceramic oxides: A precursor manufacturer’s perspective. In Proceedings of the Twelfth European Conference on Chemical Vapour Deposition, Sitges, Spain, 10–15 September 1999; pp. 169–179. [Google Scholar]
- Kuprenaite, S.; Margueron, S.; Raschetti, M.; Millon, C.; Baron, T.; Daniau, W.; Ballandras, S.; Gautier, B.; Albertini, D.; Boulet, P.; et al. Effect of LiNbO3 polarity on the structural, optical and acoustic properties of epitaxial ZnO and MgxZn1−xO films. J. Phys. D Appl. Phys. 2018, 51, 484003. [Google Scholar] [CrossRef]
- Margueron, S.; Bartasyte, A.; Plausinaitiene, V.; Abrutis, A.; Boulet, P.; Kubilius, V.; Saltyte, Z. Effect of deposition conditions on the stoichiometry and structural properties of LiNbO3 thin films deposited by MOCVD. In Oxide-based Materials and Devices IV, Proceedings of SPIE OPTO, San Francisco, CA, USA, 2–7 February 2013; Teherani, F.H., Look, D.C., Rogers, D.J., Eds.; SPIE: Bellingham, WA, USA, 2013. [Google Scholar]
- Bartasyte, A.; Margueron, S.; Baron, T.; Oliveri, S.; Boulet, P. Toward high-quality epitaxial LiNbO3 and LiTaO3 thin films for acoustic and optical applications. Adv. Mater. Interfaces 2017, 4, 1600998. [Google Scholar] [CrossRef]
- Bernal, S.J.A.D.; Diaz, J.A.; Garcia, R.; Rodriguez-Izquierdo, J.M. Study of some aspects of the reactivity of La2O3 with CO2 and H2O. J. Mater. Sci. 1985, 20, 537–541. [Google Scholar] [CrossRef]
- Zhao, Y. Design of higher-k and more stable rare earth oxides as gate dielectrics for advanced CMOS devices. Materials 2012, 5, 1413–1438. [Google Scholar] [CrossRef]
- Qiao, L.; Bi, X. Direct observation of oxygen vacancy and its effect on the microstructure, electronic and transport properties of sputtered LaNiO3−δ films on Si substrates. Thin Solid Films 2010, 519, 943–946. [Google Scholar] [CrossRef]







| Deposition Parameters | PIMOCVD | APMOCVD | DLI-CVD |
|---|---|---|---|
| Substrates | LaAlO3, R-Sapphire, SrTiO3, Si | LaAlO3, R-Sapphire, Si | R-Sapphire |
| Deposition temperature (°C) | 450–750 | 500–675 | 650–750 |
| Evaporation temperature (°C) | 200–300 | – | 150–215 |
| Carrier gas | Ar + O2 | N2 + O2 | Ar + N2 + O2 |
| Total pressure (Torr) | 5–15 | 760 | 3.4 |
| Partial O2 pressure (%) | 20 | 10 | 40 |
| Solution concentration (mol/L) | 0.02 | 0.02 | 0.01 |
| Film thickness (nm) | 120–300 | 120–260 | 60–140 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuprenaite, S.; Astié, V.; Margueron, S.; Millon, C.; Decams, J.-M.; Saltyte, Z.; Boulet, P.; Plausinaitiene, V.; Abrutis, A.; Bartasyte, A. Relationship Processing–Composition–Structure–Resistivity of LaNiO3 Thin Films Grown by Chemical Vapor Deposition Methods. Coatings 2019, 9, 35. https://doi.org/10.3390/coatings9010035
Kuprenaite S, Astié V, Margueron S, Millon C, Decams J-M, Saltyte Z, Boulet P, Plausinaitiene V, Abrutis A, Bartasyte A. Relationship Processing–Composition–Structure–Resistivity of LaNiO3 Thin Films Grown by Chemical Vapor Deposition Methods. Coatings. 2019; 9(1):35. https://doi.org/10.3390/coatings9010035
Chicago/Turabian StyleKuprenaite, Sabina, Vincent Astié, Samuel Margueron, Cyril Millon, Jean-Manuel Decams, Zita Saltyte, Pascal Boulet, Valentina Plausinaitiene, Adulfas Abrutis, and Ausrine Bartasyte. 2019. "Relationship Processing–Composition–Structure–Resistivity of LaNiO3 Thin Films Grown by Chemical Vapor Deposition Methods" Coatings 9, no. 1: 35. https://doi.org/10.3390/coatings9010035
APA StyleKuprenaite, S., Astié, V., Margueron, S., Millon, C., Decams, J. -M., Saltyte, Z., Boulet, P., Plausinaitiene, V., Abrutis, A., & Bartasyte, A. (2019). Relationship Processing–Composition–Structure–Resistivity of LaNiO3 Thin Films Grown by Chemical Vapor Deposition Methods. Coatings, 9(1), 35. https://doi.org/10.3390/coatings9010035