The Effect of Transglutaminase to Improve the Quality of Either Traditional or Pectin-Coated Falafel (Fried Middle Eastern Food)
Abstract
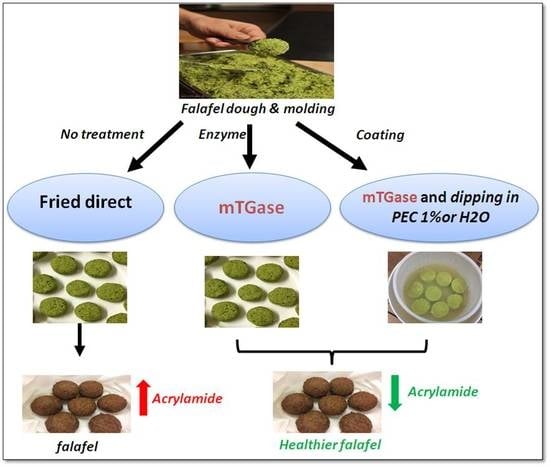
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PEC Coating Solutions
2.3. Falafel Preparation and Dipping Process
2.3.1. Falafel Dough Preparation
2.3.2. Falafel Dough Treated with TGase
2.3.3. PEC Dipping
2.4. Frying Process
2.5. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. In Vitro Gastric Digestion
2.7. ACR Standard Preparation
2.8. Extraction of ACR from the Falafel Balls
2.9. LC-MS Analysis for ACR Content of Fried Falafel
2.10. Oil Content
2.11. Water Content Analysis
2.12. Texture Profile Analysis (TPA)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Modification of the Protein Component of Falafel Balls by Means of TGase
3.2. Effect of TGase and/or 1% PEC Coating Solution on the ACR Content of Falafel Balls
3.3. Effect of TGase and/or 1% PEC Coatings on Water and Oil Content of Falafel Balls
3.4. Effect of TGase and/or 1% PEC Coatings on Texture Profile Analysis (TPA)
3.5. Effect of TGase on the Digestibility of Falafel Balls
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdullah, T. Reduction of oil uptake in deep fat fried falafel. J. Nutr. Health Food Eng. 2015, 2, 114–117. [Google Scholar] [CrossRef]
- Abu-Alruz, K. Effect of frying time and falafel balls size on fat uptake during deep fatfrying. Am.-Eur. J. Agric. Environ. Sci. 2015, 15, 1648–1654. [Google Scholar]
- Kantor, J. A history of the mideast in the humble chickpea. The New York Times, 10 July 2002. [Google Scholar]
- Al-Dmoor, H.M.; Humeid, M.A.; Alawi, M.A. Investigation of acrylamide levels in selected fried and baked foods in Jordan. J. Food Agric. Environ. 2004, 2, 157–165. [Google Scholar]
- United States Department of Agriculture. Basic Report: 16138, Falafel, Home-Prepared. Available online: https://ndb.nal.usda.gov/ndb/foods/show?ndbno=16138&fg=&man=&lfacet=&format=Abridged&count=&max=25&offset=5675&sort=c&qlookup=&rptfrm=nl&nutrient1=415&nutrient2=&nutrient3=&subset=0&totCount=7669&measureby=m (accessed on 20 May 2019).
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Food chemistry: Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- Sabbah, M.; Giosafatto, C.V.L.; Esposito, M.; Di Pierro, P.; Mariniello, L.; Porta, R. Transglutaminase cross-linked edible films and coatings for food applications. In Enzymes in Food Biotechnology, 1st ed.; Kuddus, M., Ed.; Academic Press: New York, NY, USA, 2019; pp. 369–388. [Google Scholar]
- Giosafatto, C.V.L.; Al-Asmar, A.; Mariniello, L. Transglutaminase protein substrates of food interest. In Enzymes in Food Technology: Improvement and Innovation; Kuddus, M., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 293–317. [Google Scholar]
- Pakseresht, S.; Tehrani, M.M.; Razavi, S.M.A. Optimization of low-fat set-type yoghurt: Effect of altered whey protein to casein ratio, fat content and microbial transglutaminase on rheological and sensorial properties. J. Food Sci. Technol. 2017, 54, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.; Rigby, N.; Wellner, N.; Ridout, M.; Husband, F.; Mackie, A.; Giosafatto, C.V.L. Microbial transglutaminase-mediated modification of ovalbumin. Food Hydrocoll. 2012, 26, 261–267. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Wang, H.; Shangguan, X.; Zhang, L.; Zhang, N.H.; Bansal, N. Pectin and enzyme complex modified fish scales gelatin: Rheological behavior, gel properties and nanostructure. Carbohydr. Polym. 2017, 156, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Mariniello, L.; Di Pierro, P.; Esposito, C.; Sorrentino, A.; Masi, P.; Porta, R. Preparation and mechanical properties of edible pectin-soy flour films obtained in the absence or presence of transglutaminase. J. Biotechnol. 2003, 102, 191–198. [Google Scholar] [CrossRef]
- Xing, G.; Giosafatto, C.V.L.; Rui, X.; Dong, M.; Mariniello, L. Microbial transglutaminase-mediated polymerization in the presence of lactic acid bacteria affects antigenicity of soy protein component present in bio-tofu. J. Funct. Foods 2019, 53, 292–298. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Sabbah, M.; Regalado-Gonzales, C.; Mariniello, L.; Kadivar, M.; Arabestani, A. Blend films of pectin and bitter vetch (Vicia ervilia) proteins: Properties and effect of transglutaminase. Innov. Food Sci. Emerg. Technol. 2016, 36, 245–251. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and characterization of bioplastics from grass pea flour cast in the presence of microbial transglutaminase. Coatings 2018, 8, 435. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Padmaja, N.; Bosco, S.J.D. Preservation of jujube fruits by edible Aloe vera gel coating to maintain quality and safety. Indian J. Sci. Res. Technol. 2014, 3, 79–88. [Google Scholar]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M. Natural pectin polysaccharides as edible coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef]
- Yossef, M.A. Comparison of different edible coatings materials for improvement of quality and shelf life of perishable fruits. Middle East J. Appl. Sci. 2014, 2, 416–424. [Google Scholar]
- Al-Asmar, A.; Naviglio, D.; Giosafatto, C.V.L.; Mariniello, L. Hydrocolloid-based coatings are effective at reducing acrylamide and oil content of French fries. Coatings 2018, 8, 147. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Ulfah, K.; Prangdimurti, E.; Ishikawa, Y. Effect of blanching and pectin coating as pre-frying treatments to reduce acrylamide formation in banana chips. Int. Food Res. J. 2015, 22, 936–942. [Google Scholar]
- Esposito, M.; Di Pierro, P.; Regalado-Gonzales, C.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Polyamines as new cationic plasticizers for pectin-based edible films. Carbohydr. Polym. 2016, 153, 222–228. [Google Scholar] [CrossRef]
- Marquez, G.R.; Di Pierro, P.; Esposito, M.; Mariniello, L.; Porta, R. Application of transglutaminase-crosslinked whey protein/pectin films as water barrier coatings in fried and baked foods. Food Bioprocess Technol. 2013, 7, 447–455. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nat. Cell Boil. 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bourlieu, C.; Ménard, O.; Bouzerzour, K.; Mandalari, G.; Macierzanka, A.; Mackie, A.R.; Dupont, D. Specificity of infant digestive conditions: Some clues for developing relevant in vitro models. Crit. Rev. Food Sci. Nutr. 2014, 54, 1427–1457. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, K.-W.; Du, Y.; Kong, R.; Lo, C.; Chu, I.K.; Chen, F.; Wang, M. Activities of hydrocolloids as inhibitors of acrylamide formation in model systems and fried potato strips. Food Chem. 2010, 121, 424–428. [Google Scholar] [CrossRef]
- Wang, H.; Feng, F.; Guo, Y.; Shuang, S.; Choi, M.M. HPLC-UV quantitative analysis of acrylamide in baked and deep-fried Chinese foods. J. Food Compos. Anal. 2013, 31, 7–11. [Google Scholar] [CrossRef]
- Krishna, V.N.; Meyyanathan, S.N.; Karthik, Y.; Hemnath, E.; Satiesh, K.R.; Usha, K. A simple and validated RP HPLC method for the estimation of acrylamide in potato chips. World J. Pharm. Pharm. Sci. 2014, 3, 1468–1476. [Google Scholar]
- Michalak, J.; Gujska, E.; Kuncewicz, A. RP-HPLC-DAD studies on acrylamide in cereal-based baby foods. J. Food Compos. Anal. 2013, 32, 68–73. [Google Scholar] [CrossRef]
- AOAC Official Method 960.39 Fat (Crude) or Ether Extract in Meat First Action 1960 Final Action; AOAC International: Arlington, MA, USA, 2006.
- AOAC Official Method 950.46 (39.1.02) Moisture (M); AOAC International: Arlington, MA, USA, 2006.
- Bourne, M.C. Texture profile analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Meullenet, J.F.; Lyon, B.G.; Carpenter, J.A.; Lyon, C.E. Relationship between sensory and instrumental texture profile attributes. J. Sens. Stud. 1998, 13, 77–93. [Google Scholar] [CrossRef]
- Salazar, R.; Arámbula-Villa, G.; Hidalgo, F.J.; Zamora, R. Mitigating effect of piquin pepper (Capsicum annuum L. var. Aviculare) oleoresin on acrylamide formation in potato and tortilla chips. LWT-Food Sci. Technol. 2012, 48, 261–267. [Google Scholar] [CrossRef]
- Salazar, R.; Arámbula-Villa, G.; Vázquez-Landaverde, P.A.; Hidalgo, F.J.; Zamora, R. Mitigating effect of amaranth (Amarantus hypochondriacus) protein on acrylamide formation in foods. Food Chem. 2012, 135, 2293–2298. [Google Scholar] [CrossRef]
- Sayon-Orea, C.; Bes-Rastrollo, M.; Basterra-Gortari, F.; Beunza, J.; Guallar-Castillon, P.; De La Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A. Consumption of fried foods and weight gain in a Mediterranean cohort: The SUN project. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 144–150. [Google Scholar] [CrossRef]
- Garmakhany, A.D.; Mirzaei, H.O.; Maghsudlo, Y.; Kashaninejad, M.; Jafari, S.M. Production of low fat French-fries with single and multilayer hydrocolloid coatings. J. Food Sci. Technol. 2014, 51, 1334–1341. [Google Scholar] [CrossRef]
- Mahajan, I.; Sonka, C.; Surendar, J. Study on the effective reduction of oil up-take by the application of edible hydrocolloid coatings on French fries. Int. J. Res. Eng. Adv. Technol. 2014, 2. [Google Scholar]
- Pinthus, E.J.; Weinberg, P.; Saguy, I.S. Criterion for oil uptake during deep-fat frying. J. Food Sci. 1993, 58, 204–205. [Google Scholar] [CrossRef]
- Mansour, E.H. Quality of deep-fat frying Falafel balls as influenced by various fibers and hydrocolloids. Minufiya J. Agric. Res. 2003, 28, 491–502. [Google Scholar]
- FA1005-Improving Health Properties of Food by Sharing Our Knowledge on the Digestive Process (INFOGEST). Available online: https://www.cost.eu/actions/FA1005/#tabs|Name:overview (accessed on 20 May 2019).
- Opazo-Navarrete, M.; Altenburg, M.D.; Boom, R.M.; Janssen, A.E.M. The effect of gel microstructure on simulated gastric digestion of protein gels. Food Biophys. 2018, 13, 124–138. [Google Scholar] [CrossRef]






| Falafel Type | TGase (U/g Protein) | Incubation 2 h at 37 °C | Dipping Solution |
|---|---|---|---|
| Traditional falafel | – | – | – |
| Incubated without TGase | 0 | √ | – |
| Incubated with TGase (5 U/g) | 5 | √ | – |
| Incubated with TGase (20 U/g) | 20 | √ | – |
| Dipped | – | – | Water |
| Incubated without TGase | 0 | √ | 1% PEC |
| Incubated with TGase (5 U/g) | 5 | √ | 1% PEC |
| Incubated with TGase (20 U/g) | 20 | √ | 1% PEC |
| Falafel Type | ACR Content in Spiked Sample (µg/Kg) | Recovery (%) |
|---|---|---|
| Traditional falafel | 7329 ± 188 | 100 |
| Incubated 2 h at 37 °C without TGase | 7349 ± 140 | 94.2 |
| Incubated 2 h at 37 °C with TGase 5 U/g | 6560 ± 147 a,* | 92.6 |
| Incubated 2 h at 37 °C with TGase 20 U/g | 4852 ± 217 a,* | 96.8 |
| Dipped in water | 7294 ± 348 | 102 |
| Incubated 2 h at 37 °C without TGase and dipped in 1% PEC | 3017 ± 115 a,b | 90.7 |
| Incubated 2 h at 37 °C with TGase (5 U/g) and dipped in 1% PEC | 2583 ± 65 a,b,* | 89.3 |
| Incubated 2 h at 37 °C with TGase (20 U/g) and dipped in 1% PEC | 1219 ± 83 a,b,* | 101 |
| Falafel Type | Hardness (N) | Chewiness (N·mm) | Gumminess (N) |
|---|---|---|---|
| Traditional falafel | 56.41 ± 5.50 | 184.28 ± 3.10 | 23.20 ± 1.20 |
| Incubated 2 h at 37 °C without TGase | 52.22 ± 5.30 | 180.97 ± 2.80 | 22.15 ± 1.20 |
| Incubated 2 h at 37 °C with TGase 5 U/g | 70.88 ± 3.25 a,* | 238.44 ± 2.70 a,* | Incubated 2 h at 37 °C with TGase 5 U/g |
| Incubated 2 h at 37 °C with TGase 20 U/g | 96.57 ± 4.80 a,* | 280.25 ± 15.10 a,* | 49.64 ± 3.80 a,* |
| Dipped in water | 52.18 ± 3.40 | 178.13 ± 4.10 | 21.42 ± 3.01 |
| Incubated 2 h at 37 °C without TGase and dipped in 1% PEC | 58.13 ± 4.90 | 183.23 ± 11.69 | 23.29 ± 4.50 |
| Incubated 2 h at 37 °C with TGase (5 U/g) and dipped in 1% PEC | 114.31 ± 8.20 a,b,* | 453.18 ± 11.30 a,b,* | 67.60 ± 4.50 a,b,* |
| Incubated 2 h at 37 °C with TGase (20 U/g)and dipped in 1% PEC | 136.07 ± 12.28 a,b,* | 518.50 ± 18.05 a,b,* | 78.24 ± 2.01 a,b,* |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asmar, A.; Giosafatto, C.V.L.; Panzella, L.; Mariniello, L. The Effect of Transglutaminase to Improve the Quality of Either Traditional or Pectin-Coated Falafel (Fried Middle Eastern Food). Coatings 2019, 9, 331. https://doi.org/10.3390/coatings9050331
Al-Asmar A, Giosafatto CVL, Panzella L, Mariniello L. The Effect of Transglutaminase to Improve the Quality of Either Traditional or Pectin-Coated Falafel (Fried Middle Eastern Food). Coatings. 2019; 9(5):331. https://doi.org/10.3390/coatings9050331
Chicago/Turabian StyleAl-Asmar, Asmaa, C. Valeria L. Giosafatto, Lucia Panzella, and Loredana Mariniello. 2019. "The Effect of Transglutaminase to Improve the Quality of Either Traditional or Pectin-Coated Falafel (Fried Middle Eastern Food)" Coatings 9, no. 5: 331. https://doi.org/10.3390/coatings9050331
APA StyleAl-Asmar, A., Giosafatto, C. V. L., Panzella, L., & Mariniello, L. (2019). The Effect of Transglutaminase to Improve the Quality of Either Traditional or Pectin-Coated Falafel (Fried Middle Eastern Food). Coatings, 9(5), 331. https://doi.org/10.3390/coatings9050331