Application of Activated Carbon Adsorbents Prepared from Prickly Pear Fruit Seeds and a Conductive Polymer Matrix to Remove Congo Red from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
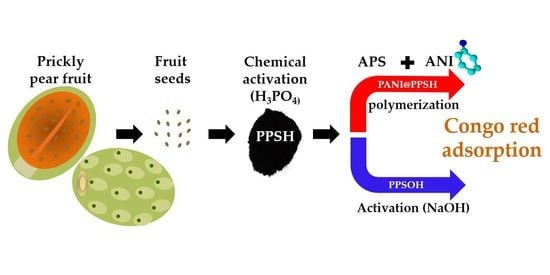
2.3. Adsorbents’ Preparation
2.3.1. Carbon Preparation
2.3.2. PANI@PPSH Preparation
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Characterization and Structural Analyses
3.2. Adsorption Assessments
3.2.1. Influence of pH
3.2.2. Contact Time Study
3.2.3. Adsorption Kinetics
3.3. Adsorption Isotherms
3.4. Adsorption Thermodynamics
3.5. Adsorption Mechanism
3.6. Stability and Recyclability of Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vallinayagam, S.; Rajendran, K.; Lakkaboyana, S.K.; Soontarapa, K.; Remya, R.R.; Sharma, V.K.; Kumar, V.; Venkateswarlu, K.; Koduru, J.R. Recent developments in magnetic nanoparticles and nano-composites for wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 106553. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.; Shan, C.; Yan, X.; Chen, H.; Pan, B. Occurrence and transformation of phosphonates in textile dyeing wastewater along full-scale combined treatment processes. Water Res. 2020, 184, 116173. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, A.; Udomsin, J.; Tsai, H.-C.; Sivakumar, M.; Hu, C.-C.; Wang, C.-F.; Hung, W.-S.; Lai, J.-Y. Special wettable underwater superoleophobic material for effective simultaneous removal of high viscous insoluble oils and soluble dyes from wastewater. J. Memb. Sci. 2020, 603, 118026. [Google Scholar] [CrossRef]
- Solangi, N.H.; Kumar, J.; Mazari, S.A.; Ahmed, S.; Fatima, N.; Mubarak, N.M. Development of fruit waste derived bio-adsorbents for wastewater treatment: A review. J. Hazard. Mater. 2021, 416, 125848. [Google Scholar] [CrossRef]
- Yang, L.; Zhan, Y.; Yu, R.; Lan, J.; Shang, J.; Dou, B.; Liu, H.; Zou, R.; Lin, S. Facile and scalable fabrication of antibacterial CO2-Responsive cotton for ultrafast and controllable removal of anionic dyes. ACS Appl. Mater. Interfaces 2021, 13, 2694–2709. [Google Scholar] [CrossRef]
- Malik, A.; Nath, M. Synthesis of Ag/ZIF-7 by immobilization of Ag nanoparticles onto ZIF-7 microcrystals: A heterogeneous catalyst for the reduction of nitroaromatic compounds and organic dyes. J. Environ. Chem. Eng. 2020, 8, 104547. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, S.; He, Y.; Fan, Y.J.; Zhang, L.; Ma, J.; Hou, R.; Chen, L.; Chen, J. A photo-Fenton self-cleaning membrane based on NH2-MIL-88B (Fe) and graphene oxide to improve dye removal performance. J. Memb. Sci. 2021, 626, 119192. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, Z.; Wang, Z.; Liu, Y.; He, Z.; Cao, A.; Zhou, L.; Zhu, L.; Zhao, S. Super-adsorptive and photo-regenerable carbon nanotube based membrane for highly efficient water purification. J. Memb. Sci. 2021, 621, 119000. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, X.; Zhu, J.; Song, Q.; Yang, G.; Zhang, Y.; Van der Bruggen, B. Self-cleaning loose nanofiltration membranes enabled by photocatalytic Cu-triazolate MOFs for dye/salt separation. J. Memb. Sci. 2021, 623, 119058. [Google Scholar] [CrossRef]
- Lakkaboyana, S.K.; Soontarapa, K.; Kumar, V.; Marella, R.K.; Kannan, K. Preparation of novel chitosan polymeric nanocomposite as an efficient material for the removal of Acid Blue 25 from aqueous environment. Int. J. Biol. Macromol. 2021, 168, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Sun, R.; Song, B.; Sun, Q.; Peng, P.; She, D. Preparation of nitrogen-doped porous carbon material by a hydrothermal-activation two-step method and its high-efficiency adsorption of Cr(VI). J. Hazard. Mater. 2020, 387, 121987. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Huang, D.D.; Wu, Y.P.; Zhao, J.; Liu, X.; Dong, W.W.; Li, S.; Li, D.S.; Li, J.R. In Situ Synthesis of Nano CuS-Embedded MOF Hierarchical Structures and Application in Dye Adsorption and Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2019, 2, 5698–5706. [Google Scholar] [CrossRef]
- Deng, J.; Chen, L.; Hong, S.; Lian, H. UZnCl2-DES assisted synthesis of phenolic resin-based carbon aerogels for capacitors. J. Porous Mater. 2020, 27, 789–800. [Google Scholar] [CrossRef]
- Díez, N.; Ferrero, G.A.; Sevilla, M.; Fuertes, A.B. A sustainable approach to hierarchically porous carbons from tannic acid and their utilization in supercapacitive energy storage systems. J. Mater. Chem. A 2019, 7, 14280–14290. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Sun, Y.; Li, X.; Hu, Z.; Worasuwannarak, N.; Liu, H.; Hu, H.; Luo, G.; Yao, H. Gas-pressurized torrefaction of biomass wastes: Co-gasification of gas-pressurized torrefied biomass with coal. Bioresour. Technol. 2020, 321, 124505. [Google Scholar] [CrossRef] [PubMed]
- Waribam, P.; Ngo, S.D.; Tran, T.T.V.; Kongparakul, S.; Reubroycharoen, P.; Chanlek, N.; Wei, L.; Zhang, H.; Guan, G.; Samart, C. Waste biomass valorization through production of xylose-based porous carbon microspheres for supercapacitor applications. Waste Manag. 2020, 105, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Tang, J.; Pol, V.G.; Lee, K.B. Pollen-derived porous carbon by KOH activation: Effect of physicochemical structure on CO2 adsorption. J. CO2 Util. 2019, 29, 146–155. [Google Scholar] [CrossRef]
- Dai, C.; Wan, J.; Yang, J.; Qu, S.; Jin, T.; Ma, F.; Shao, J. H3PO4 solution hydrothermal carbonization combined with KOH activation to prepare argy wormwood-based porous carbon for high-performance supercapacitors. Appl. Surf. Sci. 2018, 444, 105–117. [Google Scholar] [CrossRef]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, B.; Seliem, M.K.; Lamine, A.B.; Belmabrouk, H.; Bajahzar, A.; Petriciolet, A.B.; et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Netto, M.S.; Georgin, J.; Dotto, G.L.; Shayeb, M.K.A.; Belmabrouk, H.; Petriciolet, A.B.; Li, Z. Preparation and characterization of a novel mountain soursop seeds powder adsorbent and its application for the removal of crystal violet and methylene blue from aqueous solutions. Chem. Eng. J. 2020, 391, 123617. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Georgin, J.; Bajahzar, A.; Belmabrouk, H.; Lamine, A.B.; Petriciolet, A.B.; Li, Z. Adsorption of crystal violet on biomasses from pecan nutshell, para chestnut husk, araucaria bark and palm cactus: Experimental study and theoretical modeling via monolayer and double layer statistical physics models. Chem. Eng. J. 2019, 378, 122101. [Google Scholar] [CrossRef]
- Amran, F.; Zaini, M.A.A. Valorization of Casuarina empty fruit-based activated carbons for dyes removal—Activators, isotherm, kinetics and thermodynamics. Surf. Interfaces 2021, 25, 101277. [Google Scholar] [CrossRef]
- Chakraborty, S.; Farida, J.J.; Simon, R.; Kasthuri, S.; Mary, N.L. Averrhoe carrambola fruit extract assisted green synthesis of zno nanoparticles for the photodegradation of congo red dye. Surf. Interfaces 2020, 19, 100488. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, J.Á.; Ochoa-Velasco, C.E. Figo da india—Opuntia spp. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S.B.T.-E.F., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 187–201. ISBN 978-0-12-803138-4. [Google Scholar]
- Piga, A.G. Cactus pear: A fruit of nutraceutical and functional importance. J. Prof. Assoc. Cactus Dev. 2004, 6, 9–22. [Google Scholar]
- Al Juhaimi, F.; Ghafoor, K.; Uslu, N.; Mohamed Ahmed, I.A.; Babiker, E.E.; Özcan, M.M.; Fadimu, G.J. The effect of harvest times on bioactive properties and fatty acid compositions of prickly pear (Opuntia ficus-barbarica A. Berger) fruits. Food Chem. 2020, 303, 125387. [Google Scholar] [CrossRef] [PubMed]
- Saenz, C. Processing technologies: An alternative for cactus pear (Opuntia spp.) fruits and cladodes. J. Arid. Environ. 2000, 46, 209–225. [Google Scholar] [CrossRef] [Green Version]
- Özcan, M.M.; Al Juhaimi, F.Y. Nutritive value and chemical composition of prickly pear seeds (Opuntia ficus indica L.) growing in Turkey. Int. J. Food Sci. Nutr. 2011, 62, 533–536. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; El Makhfouk, M.; Qourzal, S. Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficus indica) cladodes. J. Environ. Chem. Eng. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Peláez-Cid, A.A.; Velázquez-Ugalde, I.; Herrera-González, A.M.; García-Serrano, J. Textile dyes removal from aqueous solution using Opuntia ficus-indica fruit waste as adsorbent and its characterization. J. Environ. Manag. 2013, 130, 90–97. [Google Scholar] [CrossRef]
- Bekhoukh, A.; Moulefera, I.; Zeggai, F.Z.; Benyoucef, A.; Bachari, K. Anionic Methyl Orange Removal from Aqueous Solutions by Activated Carbon Reinforced Conducting Polyaniline as Adsorbent: Synthesis, Characterization, Adsorption Behavior, Regeneration and Kinetics Study. Available online: https://link.springer.com/article/10.1007/s10924-021-02248-6 (accessed on 30 October 2021).
- Mahi, O.; Khaldi, K.; Belardja, M.S.; Belmokhtar, A.; Benyoucef, A. Development of a New Hybrid Adsorbent from Opuntia ficus indica NaOH-Activated with PANI-Reinforced and Its Potential Use in Orange-G Dye Removal. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2095–2104. [Google Scholar] [CrossRef]
- Bekhti, M.; Belardja, M.S.; Lafjah, M.; Chouli, F.; Benyoucef, A. Enhanced tailored of thermal stability, optical and electrochemical properties of PANI matrix containing Al2O3 hybrid materials synthesized through in situ polymerization. Polym. Compos. 2020, 42, 6–14. [Google Scholar] [CrossRef]
- Nepomuceno, N.C.; Seixas, A.A.A.; Medeiros, E.S.; Mélo, T.J.A. Evaluation of conductivity of nanostructured polyaniline/cellulose nanocrystals (PANI/CNC) obtained via in situ polymerization. J. Solid State Chem. 2021, 302, 122372. [Google Scholar] [CrossRef]
- Hsini, A.; Naciri, Y.; Laabd, M.; El Ouardi, M.; Ajmal, Z.; Lakhmiri, R.; Boukherroub, R.; Albourine, A. Synthesis and characterization of arginine-doped polyaniline/walnut shell hybrid composite with superior clean-up ability for chromium (VI) from aqueous media: Equilibrium, reusability and process optimization. J. Mol. Liq. 2020, 316, 113832. [Google Scholar] [CrossRef]
- Hsini, A.; Essekri, A.; Aarab, N.; Laabd, M.; Ait Addi, A.; Lakhmiri, R.; Albourine, A. Elaboration of novel polyaniline@Almond shell biocomposite for effective removal of hexavalent chromium ions and Orange G dye from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 15245–15258. [Google Scholar] [CrossRef]
- Sahnoun, S.; Boutahala, M. Adsorption removal of tartrazine by chitosan/polyaniline composite: Kinetics and equilibrium studies. Int. J. Biol. Macromol. 2018, 114, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Imgharn, A.; Ighnih, H.; Hsini, A.; Naciri, Y.; Laabd, M.; Kabli, H.; Elamine, M.; Lakhmiri, R.; Souhail, B.; Albourine, A. Synthesis and characterization of polyaniline-based biocomposites and their application for effective removal of Orange G dye using adsorption in dynamic regime. Chem. Phys. Lett. 2021, 778, 138811. [Google Scholar] [CrossRef]
- Toumi, I.; Djelad, H.; Chouli, F.; Benyoucef, A. Synthesis of PANI@ZnO Hybrid Material and Evaluations in Adsorption of Congo Red and Methylene Blue Dyes: Structural Characterization and Adsorption Performance. J. Inorg. Organomet. Polym. Mater. 2021, 32, 112–121. [Google Scholar] [CrossRef]
- Belhajjia, C.; Abid, A.; Msaad, A.; Labaali, Z.; Zouhri, A. Synthesis, characterization and adsorption of Malachite green dye using novel materiel produced from Opuntia ficus indica. Mater. Today Proc. 2021, 37, 4001–4006. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Ray, S.K. Adsorption of industrial dyes by semi-IPN hydrogels of Acrylic copolymers and sodium alginate. J. Ind. Eng. Chem. 2015, 22, 92–102. [Google Scholar] [CrossRef]
- Mannai, F.; Elhleli, H.; Ammar, M.; Passas, R.; Elaloui, E.; Moussaoui, Y. Green process for fibrous networks extraction from Opuntia (Cactaceae): Morphological design, thermal and mechanical studies. Ind. Crops Prod. 2018, 126, 347–356. [Google Scholar] [CrossRef]
- Pouget, J.P.; Jozefowicz, M.E.; Epstein, A.J.; Tang, X.; MacDiarmid, A.G. X-ray structure of polyaniline. Macromolecules 1991, 24, 779–789. [Google Scholar] [CrossRef]
- Gupta, S.P.; Nishad, H.H.; Chakane, S.D.; Gosavi, S.W.; Late, D.J.; Walke, P.S. Phase transformation in tungsten oxide nanoplates as a function of post-annealing temperature and its electrochemical influence on energy storage. Nanoscale Adv. 2020, 2, 4689–4701. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Wang, N.; Haque, M.; Naboka, O.; Flygare, M.; Svensson, K.; Gatenholm, P.; Liu, J.; Enoksson, P. Cellulose-derived carbon nanofibers/graphene composite electrodes for powerful compact supercapacitors. RSC Adv. 2017, 7, 45968–45977. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.; Zhang, Z.; Tao, J.; Zhang, D.; Chen, Y.; Lin, H. Synthesis of a poly(amidoxime-hydroxamic acid) cellulose derivative and its application in heavy metal ion removal. RSC Adv. 2017, 7, 27787–27795. [Google Scholar] [CrossRef] [Green Version]
- Habibi, M.K.; Rafiaei, S.M.; Alhaji, A.; Zare, M. Synthesis of ZnFe2O4: 1 wt% Ce3+/Carbon fibers composite and investigation of its adsorption characteristic to remove Congo red dye from aqueous solutions. J. Alloys Compd. 2022, 890, 161901. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, A.H.; Sornalingam, K. Sorptive removal of phenolic endocrine disruptors by functionalized biochar: Competitive interaction mechanism, removal efficacy and application in wastewater. Chem. Eng. J. 2018, 335, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z. Chem. Ind. Kolloide 1898, 2, 4. [Google Scholar]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Duan, R.; Fedler, C.B.; Jiao, X. Adsorption of pyridine from aqueous solutions onto polyaluminium chloride and anionic polyacrylamide water treatment residuals. Water. Sci. Technol. 2021, 83, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.O.; Kumar, R.; Ansari, S.A.; Ansari, S.P.; Barakat, M.A.; Alshahrie, A.; Cho, M.H. Anion selective pTSA doped polyaniline@graphene oxide-multiwalled carbon nanotube composite for Cr(VI) and Congo red adsorption. J. Colloid Interface Sci. 2017, 496, 407–415. [Google Scholar] [CrossRef]
- González-López, M.E.; Laureano-Anzaldo, C.M.; Pérez-Fonseca, A.A.; Gómez, C.; Robledo-Ortíz, J.R. Congo red adsorption with cellulose-graphene nanoplatelets beads by differential column batch reactor. J. Environ. Chem. Eng. 2021, 9, 105029. [Google Scholar] [CrossRef]
- Mane, V.S.; Vijay Babu, P.V. Kinetic and equilibrium studies on the removal of Congo red from aqueous solution using Eucalyptus wood (Eucalyptus globulus) saw dust. J. Taiwan Inst. Chem. Eng. 2013, 44, 81–88. [Google Scholar] [CrossRef]
- Somasekhara Reddy, M.C.; Sivaramakrishna, L.; Varada Reddy, A. The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. J. Hazard. Mater. 2012, 203, 118–127. [Google Scholar] [CrossRef]
- Tor, A.; Cengeloglu, Y. Removal of congo red from aqueous solution by adsorption onto acid activated red mud. J. Hazard. Mater. 2006, 138, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Zulfikar, M.A.; Setiyanto, H.; Rusnadi; Solakhudin, L. Rubber seeds (Hevea brasiliensis): An adsorbent for adsorption of Congo red from aqueous solution. Desalin. Water Treat. 2014, 56, 2976–2987. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]
- Chan, S.-L.; Tan, Y.P.; Abdullah, A.H.; Ong, S.-T. Equilibrium, kinetic and thermodynamic studies of a new potential biosorbent for the removal of Basic Blue 3 and Congo Red dyes: Pineapple (Ananas comosus) plant stem. J. Taiwan Inst. Chem. Eng. 2016, 61, 306–315. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Calderon, J.; Marpu, S.B.; Omary, M.A.; Shi, S.Q. Mesoporous activated carbon as a green adsorbent for the removal of heavy metals and Congo red: Characterization, adsorption kinetics, and isotherm studies. J. Contam. Hydrol. 2021, 243, 103869. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, L.; Xian, Q.; Shen, F.; Wu, J.; Zhang, Y. Removal of Congo red and methylene blue from aqueous solutions by vermicompost-derived biochars. PLoS ONE 2016, 11, e0154562. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zheng, H.; Xiong, Z.; Zhao, R.; Liu, Y.; Zhao, C.; Zheng, C. Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions. Chem. Eng. J. 2020, 392, 123706. [Google Scholar] [CrossRef]
- Masoudian, N.; Rajabi, M.; Ghaedi, M. Titanium oxide nanoparticles loaded onto activated carbon prepared from bio-waste watermelon rind for the efficient ultrasonic-assisted adsorption of congo red and phenol red dyes from wastewaters. Polyhedron 2019, 173, 114105. [Google Scholar] [CrossRef]
- Abbas, M.; Trari, M. Kinetic, equilibrium and thermodynamic study on the removal of Congo Red from aqueous solutions by adsorption onto apricot stone. Process Saf. Environ. Prot. 2015, 98, 424–436. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Fang, J.; Zhang, M.; Chen, H.; Zhou, Y.; Creamer, A.E.; Sun, Y.; Yang, L. Characterization and environmental applications of clay–biochar composites. Chem. Eng. J. 2014, 242, 136–143. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef] [Green Version]
- Namasivayam, C.; Kavitha, D. Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dye. Pigment. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A. Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. J. Hazard. Mater. 2007, 147, 979–985. [Google Scholar] [CrossRef]
- Krishna, L.S.; Reddy, A.S.; Muralikrishna, A.; Zuhairi, W.Y.W.; Osman, H.; Reddy, A.V. Utilization of the agricultural waste (Cicer arientinum Linn fruit shell biomass) as biosorbent for decolorization of Congo red. Desalin. Water Treat. 2015, 56, 2181–2192. [Google Scholar] [CrossRef]
- Moreno-López, J.C.; Pérez Paz, A.; Gottardi, S.; Solianyk, L.; Li, J.; Monjas, L.; Hirsch, A.K.H.; Mowbray, D.J.; Stöhr, M. Unveiling Adatoms in On-Surface Reactions: Combining Scanning Probe Microscopy with van’t Hoff Plots. J. Phys. Chem. C 2021, 125, 9847–9854. [Google Scholar] [CrossRef]
- Tay, S.Y.; Wong, V.L.; Lim, S.S.; Teo, I.L.R. Adsorption equilibrium, kinetics and thermodynamics studies of anionic methyl orange dye adsorption using chitosan-calcium chloride gel beads. Chem. Eng. Commun. 2021, 208, 708–726. [Google Scholar] [CrossRef]
- Mydul Islam, A.K.M.; Hwang, J.I.; Lee, S.E.; Kim, J.E. Comparative study of carbon black and activated carbon adsorbents for removal of carbofuran from aqueous solution. Desalin. Water Treat. 2016, 57, 21512–21523. [Google Scholar] [CrossRef]
- Shabandokht, M.; Binaeian, E.; Tayebi, H. Adsorption of food dye Acid red 18 onto polyaniline-modified rice husk composite: Isotherm and kinetic analysis. Desalin. Water Treat. 2016, 57, 27638–27650. [Google Scholar] [CrossRef]
- Karami, K.; Beram, S.M.; Bayat, P.; Siadatnasab, F.; Ramezanpour, A. A novel nanohybrid based on metal-organic framework MIL101 -Cr/PANI/Ag for the adsorption of cationic methylene blue dye from aqueous solution. J. Mol. Struct. 2022, 1247, 131352. [Google Scholar] [CrossRef]
- Pete, S.; Kattil, R.A.; Thomas, L. Polyaniline-multiwalled carbon nanotubes (PANI-MWCNTs) composite revisited: An efficient and reusable material for methyl orange dye removal. Diam. Relat. Mater. 2021, 117, 108455. [Google Scholar] [CrossRef]







| Materials | SBET (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) | pHPZC |
|---|---|---|---|---|
| PANI | 42.79 | 1.81 | 28.52 | 5.1 |
| PPSH | 29.25 | 2.34 | 36.77 | 6.9 |
| PPSOH | 20.21 | 2.21 | 37.55 | 9.2 |
| PANI@PPSH | 51.28 | 2.85 | 40.94 | 6.7 |
| Models | Constants | PPSH | PPSOH | PANI@PPSH |
|---|---|---|---|---|
| PFO | k1 (min−1) | 0.039 | 0.028 | 0.015 |
| Qeq.Cal (/mg·g−1) | 10.06 | 10.67 | 3.87 | |
| R 2 | 0.83 | 0.84 | 0.75 | |
| PSO | k2.ads (g·mg−1·min−1) | 0.0160 | 0.0149 | 0.0103 |
| Qeq.Cal (mg·g−1) | 9.71 | 8.18 | 17.88 | |
| R 2 | 0.99 | 0.98 | 0.99 | |
| Intraparticle diffusion | ki (g·mg−1·min−1) | 0.227 | 0.225 | 0.121 |
| C (mg·g−1) | −1.72 | −2.49 | −2.52 | |
| R 2 | 0.81 | 0.82 | 0.66 |
| Adsorbents | Constants | PPSH | PPSOH | PANI@PPSH |
|---|---|---|---|---|
| Langmuir | Qm (mg·g−1) | 21.83 | 13.57 | 27.10 |
| KL (L·mg−1) | 0.051 | 0.104 | 0.076 | |
| RL | 0.474 | 0.415 | 0.327 | |
| R 2 | 0.726 | 0.864 | 0.826 | |
| Freundlich | KF (mg1−1/nL1/ng−1) | 0.925 | 1.290 | 1.716 |
| n | 1.30 | 1.65 | 1.43 | |
| R 2 | 0.977 | 0.917 | 0.930 | |
| Temkin | B | 2.997 | 5.043 | 4.103 |
| AT (L·g−1) | 1.589 | 0.806 | 11.17 | |
| bT (J·mol−1) | 826.57 | 491.24 | 603.79 | |
| R 2 | 0.906 | 0.994 | 0.914 |
| Adsorbents | Adsorption Efficiency | pH | C0 of CR (mg·L−1) | Ref. |
|---|---|---|---|---|
| Sawdust | 31.2 | 7.0 | 20 | [55] |
| Jujuba seeds | 55.56 | 2.0 | 25 | [56] |
| Activated red mud | 7.08 | 7.0 | // | [57] |
| Rubber seeds | 9.82 | 6.0 | // | [58] |
| Cashew nutshell | 5.14 | 2.0 | // | [59] |
| Pineapple plant stem | 11.97 | 4.0 | 50 | [60] |
| Raw pinecone | 10.28 | 5.8 | // | [61] |
| Kenaf Self-activation | 14.16 | 6.95 | 1000 | [62] |
| Biochar | 11.00 | 7.5 | 1000 | [63] |
| FS@CS-PAA | 19.72 | 5.0 | 50 | [64] |
| TiO2–NPs–ACWR | 17.00 | 4.3 | 10 | [65] |
| Apricot stone | 23.42 | 13 | 100 | [66] |
| Clay–biochar composite | 11.9 | 5.7 | 20 | [67] |
| Pine bark-derived char | 3.92 | 7.0 | 5 | [68] |
| Activated carbon (Coir pith) | 6.72 | 6.3 | 40 | [69] |
| Chitosan/Montmorillonite | 12.70 | 3.0 | 200 | [70] |
| Bengal gram fruit shell (SP) | 22.22 | 6.95 | 50 | [71] |
| PANI@PPSH | 17.14 | 6.5 | 20 | This work |
| Adsorbents | T (K) | ΔG (kJ·mol−1) | ΔH (kJ·mol−1) | ΔS (kJ·mol−1) |
|---|---|---|---|---|
| PPSH | 298 | −0.30 | 23.54 | 0.080 |
| 308 | −1.10 | |||
| 318 | −1.90 | |||
| 328 | −2.70 | |||
| PPSOH | 298 | 1.012 | 10.25 | 0.031 |
| 308 | 0.702 | |||
| 318 | 0.392 | |||
| 328 | 0.082 | |||
| PANI@PPSH | 298 | −4.878 | 20.75 | 0.086 |
| 308 | −5.738 | |||
| 318 | −6.598 | |||
| 328 | −7.458 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahreche, S.; Moulefera, I.; El Kebir, A.; Sabantina, L.; Kaid, M.; Benyoucef, A. Application of Activated Carbon Adsorbents Prepared from Prickly Pear Fruit Seeds and a Conductive Polymer Matrix to Remove Congo Red from Aqueous Solutions. Fibers 2022, 10, 7. https://doi.org/10.3390/fib10010007
Lahreche S, Moulefera I, El Kebir A, Sabantina L, Kaid M, Benyoucef A. Application of Activated Carbon Adsorbents Prepared from Prickly Pear Fruit Seeds and a Conductive Polymer Matrix to Remove Congo Red from Aqueous Solutions. Fibers. 2022; 10(1):7. https://doi.org/10.3390/fib10010007
Chicago/Turabian StyleLahreche, Saadia, Imane Moulefera, Abdelkader El Kebir, Lilia Sabantina, M’hamed Kaid, and Abdelghani Benyoucef. 2022. "Application of Activated Carbon Adsorbents Prepared from Prickly Pear Fruit Seeds and a Conductive Polymer Matrix to Remove Congo Red from Aqueous Solutions" Fibers 10, no. 1: 7. https://doi.org/10.3390/fib10010007
APA StyleLahreche, S., Moulefera, I., El Kebir, A., Sabantina, L., Kaid, M., & Benyoucef, A. (2022). Application of Activated Carbon Adsorbents Prepared from Prickly Pear Fruit Seeds and a Conductive Polymer Matrix to Remove Congo Red from Aqueous Solutions. Fibers, 10(1), 7. https://doi.org/10.3390/fib10010007