Characterization of Developing Cotton Fibers by Confocal Raman Microscopy
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Scanning Electron Microscopy
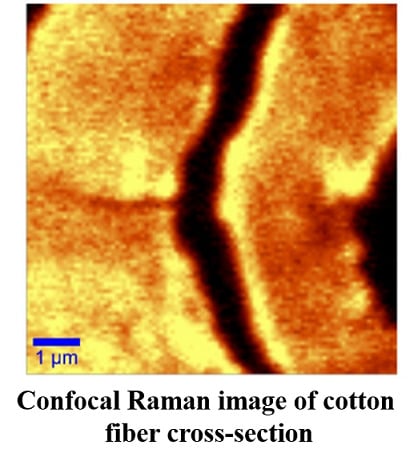
2.2.2. Confocal Raman Microscopy (CRM)
2.2.3. X-Ray Diffraction
3. Results and Discussion



| Wavenumbers (cm−1) | Relative intensity (a.u.) | Assignment [5,6,7,21,22] | ||
|---|---|---|---|---|
| 56 dpa | 27 dpa | 21 dpa | – | |
| 2897 | 2252 | 2287 | 1015 | CH, CH2 stretching |
| 2736 | 236 | 270 | 97 | CH Methine |
| 1481 | 236 | 234 | 218 | Correlated to cellulose crystalline |
| 1461 | 250 | 279 | 230 | Correlated to amorphous cellulose |
| 1379 | 774 | 685 | 501 | CH2, HCC, HCO, COH |
| 1158 | 763 | 681 | 436 | CC, CO ring breathing, asymmetric stretching |
| 1124 | 1135 | 1041 | 825 | COC glycosidic linkage, symmetric stretching |
| 1099 | 1000 | 1000 | 1000 | COC glycosidic, ring breathing |
| 974 | 264 | 237 | 137 | CH2 |
| 521 | 656 | 555 | 286 | COC glycosidic |
| 439 | 738 | 654 | 397 | CCC, CCO ring deformation |
| 383 | 1108 | 1008 | 526 | CCC, CO, CCO, ring deformation/correlated to cellulose crystallinity |
| 346 | 502 | 470 | 256 | CCC, CO, CCO, ring deformation |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gierlinger, N.; Schwanninger, M. The potential of Raman microscopy and Raman imaging in plant research. Spectr. Int. J. 2007, 21, 69–89. [Google Scholar] [CrossRef]
- Chan, K.L.A.; Kazarian, S.G.; Mavraki, A.; Williams, D.R. Fourier Transform Infrared imaging of Human hair with high spatial resolution without the use of a synchrotron. Appl. Spectrosc. 2005, 59, 149–155. [Google Scholar] [CrossRef]
- Roder, T.; Koch, G.; Sixta, H. Application of confocal Raman spectroscopy for the topochemical distribution of lignin and cellulose in plant cell walls of beech wood (Fagus sylvatica L.) compared to UV microspectrophotometry. Holzforschung 2004, 58, 480–482. [Google Scholar] [CrossRef]
- Dieing, T.; Hollricher, O. High resolution, high-speed confocal Raman imaging. Vib. Spectrosc. 2008, 48, 22–27. [Google Scholar] [CrossRef]
- Schenzel, K.; Fischer, S.; Brendler, E. New method for determining the degree of cellulose I crystallinity by means of FT Raman spectroscopy. Cellulose 2005, 12, 223–231. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Reiner, R.S.; Ralph, S.A. Determination of Cellulose I Crystallinity by FT-Raman Spectroscopy. In Proceeding of the 15th International Symposium on Wood; Fiber and Pulping Chemistry: Oslo, Norway, 2009. [Google Scholar]
- Eronen, P.; Osterberg, M.; Jaaskelainen, A.S. Effect of alkaline treatment on cellulose supramolecular structure studied with combined confocal Raman spectroscopy and atomic force microscopy. Cellulose 2009, 16, 167–178. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Cabrales, L.; Gannaway, J.; Wilkins, T.; Wells, L.W. Evaluating cell wall structure and composition of developing cotton fibers using Fourier Transform Infrared spectroscopy and thermogravimetric analysis. J. Appl. Polym. Sci. 2008, 107, 476–486. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Cabrales, L. Changes in sugar composition and cellulose content during the secondary cell wall biogenesis in cotton fibers. Cellulose 2010, 17, 153–160. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Hequet, E. Fourier transform infrared spectroscopy approach to the study of the secondary cell wall development in cotton fiber. Cellulose 2010, 17, 309–320. [Google Scholar] [CrossRef]
- Gierlinger, N.; Schwanninger, M. Chemical imaging of poplar wood cell walls by confocal Raman microscopy. Plant Physiol. 2006, 140, 1246–1254. [Google Scholar] [CrossRef]
- Agarwal, U.P. Raman imaging to investigate ultrastructure and composition of plant cell walls: distribution of lignin and cellulose in black spruce wood (Picea mariana). Planta 2006, 224, 1141–1153. [Google Scholar] [CrossRef]
- Osterberg, M.; Schmidt, U.; Jaaskelainen, A.S. Combining confocal Raman spectroscopy and atomic force microscopy to study wood extractives on cellulose surface. Colloid Surf. A Physicochem. Eng. Asp. 2006, 291, 197–201. [Google Scholar] [CrossRef]
- Schmidt, M.; Schwartzberg, A.M.; Carroll, A.; Chaibang, A.; Adams, P.D.; Schuck, P.J. Raman Imaging of cell wall polymers in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2010, 395, 521–523. [Google Scholar] [CrossRef]
- Schmidt, M.; Schwartzberg, A.M.; Perera, P.N.; Weber-Bargioni, A.; Carroll, A.; Sarkar, P.; Bosneaga, E.; Urban, J.J.; Song, J.; Balakshin, M.Y.; et al. Label-free in situ imaging of lignification in the cell wall of low lignin transgenic. Populus Trichcarpa. Planta 2009, 230, 589–597. [Google Scholar]
- Mangiante, G.; Alcouffe, P.; Burdin, B.; Gaborieau, M.; Zeno, E.; Petit-Conil, M.; Bernard, J.; Charlot, A.; Fleury, E. Green nondegrading approach to alkyne-functionalized cellulose fibers and biohybrids thereof: Synthesis and maping of the derivatization. Biomacromolecules 2013, 14, 254–263. [Google Scholar] [CrossRef]
- Song, Z.; Chen, Z.; Bian, X.; Zhou, L.; Ding, D.; Liang, H.; Zou, Y.; Wang, S.; Chen, L.; Yang, C.; et al. Alkyne-functionalized superstable graphitic nanoparticles for Raman imaging. J. Am. Chem. Soc. 2014, 136, 13558–13561. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Ethridge, D. Thermogravimetric analysis of cotton fibers: Relationships with maturity and finneness. J. Appl. Polym. Sci. 2007, 103, 3476–3482. [Google Scholar] [CrossRef]
- Kim, H.J.; Triplett, B.A. Cotton fiber growth in planta and in vitro. Models for plant cell wall elongation and cell wall biogenesis. Plant Physiol. 2001, 127, 1361–1366. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Kokot, S. Raman spectroscopic investigation of acetylation of raw cotton. Spectroc. Acta A Molec. Biomolec. Spectr. 2006, 64, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Kokot, S.; Sambi, T.J. Vibrational spectroscopic investigation of Australian cotton cellulose fibres. Part 1. A Fourier transform Raman study. Analyst 1998, 123, 633–636. [Google Scholar] [CrossRef]
- Schenzel, K.; Fischer, S. NIR FT Raman spectroscopy—A rapid analytical tool for detecting the transformation of cellulose polymorphs. Cellulose 2001, 8, 49–57. [Google Scholar] [CrossRef]
- Hu, X.-P.; Hsieh, Y.-L. Crystalline structure of developing cotton fibers. J. Polym. Sci. B Polym. Phys. 1996, 34, 1451–1459. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrales, L.; Abidi, N.; Manciu, F. Characterization of Developing Cotton Fibers by Confocal Raman Microscopy. Fibers 2014, 2, 285-294. https://doi.org/10.3390/fib2040285
Cabrales L, Abidi N, Manciu F. Characterization of Developing Cotton Fibers by Confocal Raman Microscopy. Fibers. 2014; 2(4):285-294. https://doi.org/10.3390/fib2040285
Chicago/Turabian StyleCabrales, Luis, Noureddine Abidi, and Felicia Manciu. 2014. "Characterization of Developing Cotton Fibers by Confocal Raman Microscopy" Fibers 2, no. 4: 285-294. https://doi.org/10.3390/fib2040285
APA StyleCabrales, L., Abidi, N., & Manciu, F. (2014). Characterization of Developing Cotton Fibers by Confocal Raman Microscopy. Fibers, 2(4), 285-294. https://doi.org/10.3390/fib2040285