Fabrication of a Fluorophore-Doped Cylindrical Waveguide Structure Using Elastomers for Visual Detection of Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dispersal of Fluorophore in an Elastomer Bulk
2.2. Refractive Index and Elasticity Measurement for Selecting a Core–Cladding Pair
2.3. Density and Atomic Characterizations of Selected Elastomers
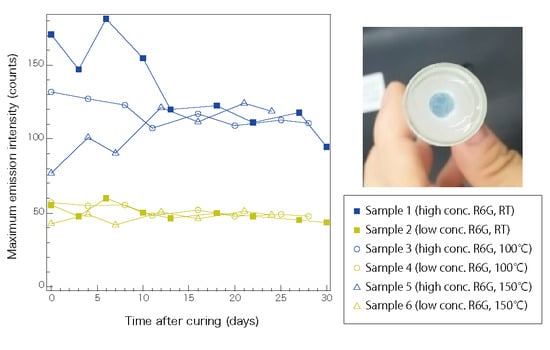
3. Results and Discussion
3.1. Emission Spectrometry of the Fluorophore-Doped Elastomer
3.2. Selecting Elastomer Pair with Similar Characteristics for Core and Cladding
3.3. Dependence of Volumetric Properties on Curing Conditions
3.4. Elemental Analysis of the Elastomer and Precursors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cimellaro, G.P.; Solari, D.; Bruneau, M. Physical Infrastructure Interdependency and Regional Resilience Index after the 2011 Tohoku Earthquake in Japan. Earthq. Eng. Struct. Dyn. 2014, 43, 1763–1784. [Google Scholar] [CrossRef]
- Matsuda, I. Verifying Vulnerability to Natural Disasters in Tokyo. J. Geogr. (Chigaku Zasshi) 2013, 122, 1070–1087. [Google Scholar] [CrossRef]
- Glisic, B.; Inaudi, D. Fibre Optic Methods for Structural Health Monitoring; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kamimura, S.; Furukawa, R. Strain Sensing Based on Radiative Emission-Absorption Mechanism Using Dye-Doped Polymer Optical Fiber. Appl. Phys. Lett. 2017, 111, 063301:1–063301:4. [Google Scholar] [CrossRef]
- Furukawa, R.; Tagaya, A.; Iwata, S.; Koike, Y. Polarization-Maintaining Mechanism of a Birefringence-Reduced Plastic Optical Fiber Fabricated Using Poly(methyl methacrylate/benzyl methacrylate) Copolymer. J. Phys. Chem. C 2008, 112, 7946–7952. [Google Scholar] [CrossRef]
- Paek, U.C.; Runk, R.B. Physical Behavior of the Neck-Down Region during Furnace Drawing of Silica Fibers. J. Appl. Phys. 1978, 49, 4417–4422. [Google Scholar] [CrossRef]
- Lotters, J.C.; Olthuis, W.; Veltink, P.H.; Bergveld, P. The Mechanical Properties of the Rubber Elastic Polymer Polydimethylsiloxane for Sensor Applications. J. Micromech. Microeng. 1997, 7, 145–147. [Google Scholar] [CrossRef]
- Zubia, J.; Arrue, J. Plastic Optical Fibers: An Introduction to Their Technological Processes and Applications. Opt. Fiber Technol. 2001, 7, 101–140. [Google Scholar] [CrossRef]
- Kurian, A.; George, N.A.; Paul, B.; Nampoori, V.P.N.; Vallabhan, C.P.G. Studies on Fluorescence Efficiency and Photodegradation of Rhodamine 6G Doped PMMA Using a Dual Beam Thermal Lens Technique. Laser Chem. 2002, 20, 99–110. [Google Scholar] [CrossRef]
- Ishigure, T.; Sato, M.; Takanashi, O.; Nihei, E.; Nyu, T.; Yamazaki, S.; Koike, Y. Formation of the Refractive Index Profile in the Graded Index Polymer Optical Fiber for Gigabit Data Transmission. J. Lightw. Technol. 1997, 15, 2095–2100. [Google Scholar] [CrossRef]







| Sample | R6G Concentration (wt.%) | Cyclopentanone Content per 10 g host (µL) | Curing Conditions |
|---|---|---|---|
| Sample 1 | 1.33 × | 300 | Room temperature, 24 h |
| Sample 2 | 2.85 × | 1400 | |
| Sample 3 | 1.33 × | 300 | 100 °C, 1 h |
| Sample 4 | 2.85 × | 1400 | |
| Sample 5 | 1.33 × | 300 | 150 °C, 1 h |
| Sample 6 | 2.85 × | 1400 |
| Elastomer | Refractive Index Given by the Supplier | Recommended Curing Conditions |
|---|---|---|
| A | 1.41 | Minimum 30 min exposure at 150 °C Minimum 24 h exposure at room temperature |
| B | 1.33 | 1 h exposure at 120 °C |
| C | 1.41 | 2000 mJ cm−2 exposure at 365 nm (over 40 mW cm−2 in intensity) |
| D | 1.51 | 1 h exposure at 100 °C 5 h exposure at 150 °C |
| Elastomer | Rubber Hardness |
|---|---|
| A | 56 |
| B | 34 |
| C | 63 |
| D | 75 |
| Core | Cladding | NA (@20 °C) | ∆RH |
|---|---|---|---|
| Elastomer A | Elastomer B | 0.46 | 22 |
| Elastomer C | Elastomer B | 0.46 | 29 |
| Elastomer D | Elastomer A | 0.56 | 19 |
| Elastomer D | Elastomer B | 0.73 | 41 |
| Elastomer D | Elastomer C | 0.56 | 12 |
| Element | Precursor 1 | Precursor 2 | Bulk 1 | Bulk 2 | Bulk 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| V | α | V | α | V | α | V | α | V | α | |
| Si | 987,247.3 | 9542 | 987,514.2 | 9550.1 | 999,758.2 | 9060.4 | 979,035.6 | 6467.6 | 978,157.1 | 7010.1 |
| Cl | - | - | - | - | - | - | 20,496.2 | 741.2 | 21,598.8 | 815 |
| S | 11,957 | 1966.5 | 11,752.6 | 1972.6 | - | - | - | - | - | - |
| Cu | 386 | 25.1 | 394.9 | 25.2 | 196.4 | 15.8 | 173.5 | 10.7 | 173.4 | 12.4 |
| Pt | 0.011 | 0.005 | ND | 0 | 0.005 | 0.004 | 0.005 | 0.002 | 0.007 | 0.003 |
| Sn | 0.006 | 0.003 | 0.007 | 0.003 | ND | 0 | 0.002 | 0.001 | ND | 0 |
| Fe | 235.7 | 45.2 | 272.6 | 45.4 | 222.3 | 20.8 | - | - | - | - |
| Element | Precursor 1 | Precursor 2 | Bulk 1 | Bulk 2 | ||||
|---|---|---|---|---|---|---|---|---|
| V | α | V | α | V | α | V | α | |
| Si | - | - | 858,158.2 | 796,383.3 | 576,938.4 | 755,208.8 | 660,888.4 | 762,579 |
| Cl | - | - | - | - | 410,817.4 | 207,882.5 | 325,871.7 | 194,472.3 |
| S | 838,817.4 | 814,858 | 135,168.5 | 191,177.9 | - | - | - | - |
| Cu | 91,121.4 | 23,179.6 | 4129.1 | 1999.6 | 5400.5 | 4391.4 | 5245.9 | 4560.8 |
| Pt | ND | 7 | ND | 0.5 | ND | 1.2 | ND | 1.2 |
| Sn | 2.452 | 6.046 | 0.066 | 0.276 | ND | 0.8 | 0.066 | 0.724 |
| Fe | - | - | - | - | 4152.2 | 7506.3 | 2650.8 | 7103.6 |
| Ni | 45,542.8 | 31,810.1 | 1887.8 | 2637.4 | 2691.5 | 5780.4 | 2588.3 | 6010.8 |
| Cd | - | - | - | - | - | - | 2091 | 6019.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirose, C.; Fukuda, N.; Sassa, T.; Ishibashi, K.; Ochiai, T.; Furukawa, R. Fabrication of a Fluorophore-Doped Cylindrical Waveguide Structure Using Elastomers for Visual Detection of Stress. Fibers 2019, 7, 37. https://doi.org/10.3390/fib7050037
Hirose C, Fukuda N, Sassa T, Ishibashi K, Ochiai T, Furukawa R. Fabrication of a Fluorophore-Doped Cylindrical Waveguide Structure Using Elastomers for Visual Detection of Stress. Fibers. 2019; 7(5):37. https://doi.org/10.3390/fib7050037
Chicago/Turabian StyleHirose, Chie, Nobuko Fukuda, Takafumi Sassa, Koji Ishibashi, Tsuyoshi Ochiai, and Rei Furukawa. 2019. "Fabrication of a Fluorophore-Doped Cylindrical Waveguide Structure Using Elastomers for Visual Detection of Stress" Fibers 7, no. 5: 37. https://doi.org/10.3390/fib7050037
APA StyleHirose, C., Fukuda, N., Sassa, T., Ishibashi, K., Ochiai, T., & Furukawa, R. (2019). Fabrication of a Fluorophore-Doped Cylindrical Waveguide Structure Using Elastomers for Visual Detection of Stress. Fibers, 7(5), 37. https://doi.org/10.3390/fib7050037