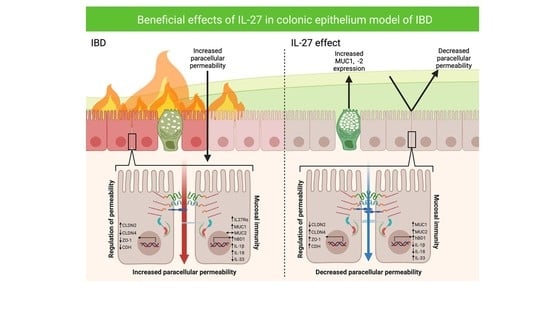
Interleukin-27 Regulates the Function of the Gastrointestinal Epithelial Barrier in a Human Tissue-Derived Organoid Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Tissue Source
2.2. Colorectal Cell Line Culture
2.3. Human Colon-Derived Epithelial Organoid Model
2.3.1. 3D Organoid Culture
2.3.2. Human Colon Organoid-Derived Monolayer Culture
2.4. Organoid Stimulation Assays and Ex Vivo Inflammatory Organoid Model
2.5. Gene Expression Analysis
2.6. Protein Extraction and Immunoblotting
2.7. Epithelial Barrier Permeability Assay
2.8. Wound Healing Assay
2.9. Proliferation Assay
2.10. Statistical Analysis
3. Results
3.1. IL-27 Regulated Permeability of the Human Gastrointestinal Epithelial Barrier Following Inflammatory Insult
3.2. IL-27 Led to Differential Expression of Key Mediators of Human Gastrointestinal Epithelial Barrier Permeability and Barrier Integrity in a Human Colon Organoid Model
3.3. IL-27 Stimulated Human Gastrointestinal Epithelial Barrier-Derived Innate Immunity
3.4. IL-27 Stimulated Gastrointestinal Epithelial Barrier Wound Restitution and Proliferation
3.5. Expression of the Receptor for IL-27 Increases with TNF Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.Y.; Söderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Hanson, M.L.; Hixon, J.A.; Li, W.; Felber, B.K.; Anver, M.R.; Stewart, C.A.; Janelsins, B.M.; Datta, S.K.; Shen, W.; McLean, M.H.; et al. Oral delivery of il-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology 2014, 146, 210–221.e13. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.H.; Andrews, C.; Hanson, M.L.; Baseler, W.A.; Anver, M.R.; Senkevitch, E.; Staniszewska, A.K.; Smith, C.; Davies, L.C.; Hixon, J.; et al. Interleukin-27 Is a Potential Rescue Therapy for Acute Severe Colitis Through Interleukin-10-Dependent, T-Cell-Independent Attenuation of Colonic Mucosal Innate Immune Responses. Inflamm. Bowel Dis. 2017, 23, 1983–1995. [Google Scholar] [CrossRef]
- Yoshida, H.; Hunter, C.A. The immunobiology of interleukin-27. Annu. Rev. Immunol. 2015, 33, 417–443. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, M.C.; Lin, L.L.; Christian, D.A.; Min, B.; Hunter, C.A.; Lu, L.F. Gut epithelial IL-27 confers intestinal immunity through the induction of intraepithelial lymphocytes. J. Exp. Med. 2021, 218, e20210021. [Google Scholar] [CrossRef]
- Porter, R.J.; Murray, G.I.; Alnabulsi, A.; Humphries, M.P.; James, J.A.; Salto-Tellez, M.; Craig, S.G.; Wang, J.M.; Yoshimura, T.; McLean, M.H. Colonic epithelial cathelicidin (LL-37) expression intensity is associated with progression of colorectal cancer and presence of CD8+ T cell infiltrate. J. Pathol. Clin. Res. 2021, 7, 495–506. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Loonen, L.M.P.; Taverne, N.; Taverne-Thiele, J.J.; Smidt, H.; Wells, J.M. Optimized procedures for generating an enhanced, near physiological 2D culture system from porcine intestinal organoids. Stem Cell Res. 2018, 28, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Lampri, E.; Fitsiou, E.; Vasileiadis, S.; Vamvakias, M.; Bardouki, H.; Goussia, A.; Malamou-Mitsi, V.; Panayiotidis, M.I.; et al. Dietary mastic oil extracted from Pistacia lentiscus var. chia suppresses tumor growth in experimental colon cancer models. Sci. Rep. 2017, 7, 3782. [Google Scholar] [CrossRef] [PubMed]
- Cormier, N.; Yeo, A.; Fiorentino, E.; Paxson, J. Optimization of the Wound Scratch Assay to Detect Changes in Murine Mesenchymal Stromal Cell Migration After Damage by Soluble Cigarette Smoke Extract. J. Vis. Exp. 2015, e53414. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Ward, F.J.; Vickers, M.A.; Stott, L.-M.; Urbaniak, S.J.; Barker, R.N. Interleukin-10–mediated regulatory T-cell responses to epitopes on a human red blood cell autoantigen. Blood 2002, 100, 4529–4536. [Google Scholar] [CrossRef] [PubMed]
- Madara, J.L.; Stafford, J. Interferon-γ directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Investig. 1989, 83, 724–727. [Google Scholar] [CrossRef]
- Schwank, G.; Andersson-Rolf, A.; Koo, B.K.; Sasaki, N.; Clevers, H. Generation of BAC Transgenic Epithelial Organoids. PLoS ONE 2013, 8, e76871. [Google Scholar] [CrossRef]
- Driehuis, E.; Clevers, H. CRISPR/Cas 9 genome editing and its applications in organoids. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G257–G265. [Google Scholar] [CrossRef]
- Wang, F.; Graham, W.V.; Wang, Y.; Witkowski, E.D.; Schwarz, B.T.; Turner, J.R. Interferon-gamma; and Tumor Necrosis Factor-alpha; Synergize to Induce Intestinal Epithelial Barrier Dysfunction by Up-Regulating Myosin Light Chain Kinase Expression. Am. J. Pathol. 2005, 166, 409–419. [Google Scholar] [CrossRef]
- Wang, F.; Schwarz, B.T.; Graham, W.V.; Wang, Y.; Su, L.; Clayburgh, D.R.; Abraham, C.; Turner, J.R. IFN-γ-Induced TNFR2 Expression Is Required for TNF-Dependent Intestinal Epithelial Barrier Dysfunction. Gastroenterology 2006, 131, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Ferrante, M.; Magro, F.; Campbell, S.; Franchimont, D.; Fidder, H.; Strid, H.; Ardizzone, S.; Veereman-Wauters, G.; Chevaux, J.-B.; et al. Results from the 2nd Scientific Workshop of the ECCO (I): Impact of mucosal healing on the course of inflammatory bowel disease. J. Crohn’s Colitis 2011, 5, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Cassinotti, A.; Duca, P.; Mazzali, C.; Penati, C.; Manes, G.; Marmo, R.; Massari, A.; Molteni, P.; Maconi, G.; et al. Mucosal Healing Predicts Late Outcomes After the First Course of Corticosteroids for Newly Diagnosed Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 483–489.e3. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L.; Burisch, J. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.; McLean, M.H.; Durum, S.K. Interleukin-27 as a Novel Therapy for Inflammatory Bowel Disease: A Critical Review of the Literature. Inflamm. Bowel Dis. 2016, 22, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, J.; Olszak, T.; Göke, B.; Blumberg, R.S.; Brand, S. A novel role for interleukin-27 (IL-27) as mediator of intestinal epithelial barrier protection mediated via differential signal transducer and activator of transcription (STAT) protein signaling and induction of antibacterial and anti-inflammatory protei. J. Biol. Chem. 2012, 287, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Suwanpradid, J.; Sanchez-Lagunes, R.; Choi, H.W.; Hoang, P.; Wang, D.; Abraham, S.N.; MacLeod, A.S. IL-27 Facilitates Skin Wound Healing through Induction of Epidermal Proliferation and Host Defense. J. Investig. Dermatol. 2017, 137, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wu, X.; Gong, Y.; Cao, J. IL-27 induces LL-37/CRAMP expression from intestinal epithelial cells: Implications for immunotherapy of Clostridioides difficile infection. Gut Microbes 2021, 13, 1968258. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: Mechanism and applications. Science 2013, 340, 1190–1194. [Google Scholar] [CrossRef]
- Porter, R.J.; Murray, G.I.; McLean, M.H. Current concepts in tumour-derived organoids. Br. J. Cancer 2020, 123, 1209–1218. [Google Scholar] [CrossRef]
- O’Connell, L.; Winter, D.C.; Aherne, C.M. The Role of Organoids as a Novel Platform for Modeling of Inflammatory Bowel Disease. Front. Pediatr. 2021, 9, 624045. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, E.; Veghini, L.; Corbo, V. Modeling Cell Communication in Cancer With Organoids: Making the Complex Simple. Front. Cell Dev. Biol. 2020, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vanuytsel, T.; Farré, R.; Verstockt, S.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Schuit, F.; Vermeire, S.; Arijs, I.; et al. Genetic and Transcriptomic Bases of Intestinal Epithelial Barrier Dysfunction in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- d’Aldebert, E.; Quaranta, M.; Sébert, M.; Bonnet, D.; Kirzin, S.; Portier, G.; Duffas, J.-P.; Chabot, S.; Lluel, P.; Allart, S.; et al. Characterization of Human Colon Organoids From Inflammatory Bowel Disease Patients. Front. Cell Dev. Biol. 2020, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Rallabandi, H.R.; Yang, H.; Oh, K.B.; Lee, H.C.; Byun, S.J.; Lee, B.R. Evaluation of Intestinal Epithelial Barrier Function in Inflammatory Bowel Diseases Using Murine Intestinal Organoids. Tissue Eng. Regen. Med. 2020, 17, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, S.K.; Signs, S.; Hu, B.; Yeu, Y.; Feng, H.; Ni, Y.; Hill, D.R.; Fisher, R.C.; Ferrandon, S.; DeHaan, R.K.; et al. Induced organoids derived from patients with ulcerative colitis recapitulate colitic reactivity. Nat. Commun. 2021, 12, 262. [Google Scholar] [CrossRef]
- Nishimura, R.; Shirasaki, T.; Tsuchiya, K.; Miyake, Y.; Watanabe, Y.; Hibiya, S.; Watanabe, S.; Nakamura, T.; Watanabe, M. Establishment of a system to evaluate the therapeutic effect and the dynamics of an investigational drug on ulcerative colitis using human colonic organoids. J. Gastroenterol. 2019, 54, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-H.; Donowitz, M. Intestinal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases. World J. Gastroenterol. 2019, 25, 4125–4147. [Google Scholar] [CrossRef] [PubMed]
- Dotti, I.; Mora-Buch, R.; Ferrer-Picón, E.; Planell, N.; Jung, P.; Masamunt, M.C.; Leal, R.F.; Martín de Carpi, J.; Llach, J.; Ordás, I.; et al. Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut 2017, 66, 2069. [Google Scholar] [CrossRef]
- Howell, K.J.; Kraiczy, J.; Nayak, K.M.; Gasparetto, M.; Ross, A.; Lee, C.; Mak, T.N.; Koo, B.-K.; Kumar, N.; Lawley, T.; et al. DNA Methylation and Transcription Patterns in Intestinal Epithelial Cells From Pediatric Patients With Inflammatory Bowel Diseases Differentiate Disease Subtypes and Associate With Outcome. Gastroenterology 2018, 154, 585–598. [Google Scholar] [CrossRef]
- Noben, M.; Verstockt, B.; de Bruyn, M.; Hendriks, N.; Van Assche, G.; Vermeire, S.; Verfaillie, C.; Ferrante, M. Epithelial organoid cultures from patients with ulcerative colitis and Crohn’s disease: A truly long-term model to study the molecular basis for inflammatory bowel disease? Gut 2017, 66, 2193. [Google Scholar] [CrossRef] [PubMed]
- Grabinger, T.; Luks, L.; Kostadinova, F.; Zimberlin, C.; Medema, J.P.; Leist, M.; Brunner, T. Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis. 2014, 5, e1228. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, R.; Watanabe, M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J. Gastroenterol. 2016, 51, 11–21. [Google Scholar] [CrossRef] [PubMed]
- McCole, D.F. IBD candidate genes and intestinal barrier regulation. Inflamm. Bowel Dis. 2014, 20, 1829–1849. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Philip Schumm, L.; Sharma, Y.; Anderson, C.A.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Irvine, E.J.; Marshall, J.K. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology 2000, 119, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, J.; Vogelsang, H.; Hübl, W.; Waldhoer, T.; Lochs, H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet 1993, 341, 1437–1439. [Google Scholar] [CrossRef]
- Fries, W.; Renda, M.C.; Lo Presti, M.A.; Raso, A.; Orlando, A.; Oliva, L.; Giofré, M.R.; Maggio, A.; Mattaliano, A.; Macaluso, A.; et al. Intestinal Permeability and Genetic Determinants in Patients, First-Degree Relatives, and Controls in a High-Incidence Area of Crohn’s Disease in Southern Italy. Am. J. Gastroenterol. 2005, 100, 2730–2736. [Google Scholar] [CrossRef]
- Vogelsang, H. Do changes in intestinal permeability predict disease relapse in Crohn’s disease? Inflamm. Bowel Dis. 2008, 14, S162–S163. [Google Scholar] [CrossRef]
- Čužić, S.; Antolić, M.; Ognjenović, A.; Stupin-Polančec, D.; Petrinić Grba, A.; Hrvačić, B.; Dominis Kramarić, M.; Musladin, S.; Požgaj, L.; Zlatar, I.; et al. Claudins: Beyond Tight Junctions in Human IBD and Murine Models. Front. Pharmacol. 2021, 12, 682614. [Google Scholar] [CrossRef]
- Acovic, A.; Gazdic, M.; Jovicic, N.; Harrell, C.R.; Fellabaum, C.; Arsenijevic, N.; Volarevic, V. Role of indoleamine 2,3-dioxygenase in pathology of the gastrointestinal tract. Therap. Adv. Gastroenterol. 2018, 11, 1756284818815334. [Google Scholar] [CrossRef] [PubMed]
- Carbotti, G.; Barisione, G.; Airoldi, I.; Mezzanzanica, D.; Bagnoli, M.; Ferrero, S.; Petretto, A.; Fabbi, M.; Ferrini, S. IL-27 induces the expression of IDO and PD-L1 in human cancer cells. Oncotarget 2015, 6, 43267–43280. [Google Scholar] [CrossRef] [PubMed]
- Ferdinande, L.; Demetter, P.; Perez-Novo, C.; Waeytens, A.; Taildeman, J.; Rottiers, I.; Rottiers, P.; De Vos, M.; Cuvelier, C.A. Inflamed intestinal mucosa features a specific epithelial expression pattern of indoleamine 2,3-dioxygenase. Int. J. Immunopathol. Pharmacol. 2008, 21, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Biancone, L.; Armuzzi, A.; Scribano, M.L.; Castiglione, F.; D’Incà, R.; Orlando, A.; Papi, C.; Daperno, M.; Vecchi, M.; Riegler, G.; et al. Cancer Risk in Inflammatory Bowel Disease: A 6-Year Prospective Multicenter Nested Case–Control IG-IBD Study. Inflamm. Bowel Dis. 2020, 26, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Ye, L.; Wang, O.; Huang, G.; Yang, F.; Liu, Y.; Dong, S. IL-27 rs153109 polymorphism increases the risk of colorectal cancer in Chinese Han population. Onco. Targets. Ther. 2015, 8, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Kourko, O.; Seaver, K.; Odoardi, N.; Basta, S.; Gee, K. IL-27, IL-30, and IL-35: A Cytokine Triumvirate in Cancer. Front. Oncol. 2019, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.L.; Letteri, R.; Chan-Seng, D.; Kumar, S.; Rivera-Cruz, C.M.; Emrick, T.S. Reengineering Tumor Microenvironment with Sequential Interleukin Delivery. Bioengineering 2021, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Lu, S.; Lai, L.; Xie, Y.; He, J.; Xue, Y.; Xiao, P.; Pan, T.; Chen, L.; Liu, Y.; et al. Protective function of interleukin 27 in colitis-associated cancer via suppression of inflammatory cytokines in intestinal epithelial cells. Oncoimmunology 2017, 6, e1268309. [Google Scholar] [CrossRef] [PubMed]
- Daulagala, A.C.; Bridges, M.C.; Kourtidis, A. E-cadherin Beyond Structure: A Signaling Hub in Colon Homeostasis and Disease. Int. J. Mol. Sci. 2019, 20, 2756. [Google Scholar] [CrossRef] [PubMed]






| Relative Protein Expression | ||||
|---|---|---|---|---|
| Claudin-2 | Claudin-4 | Occludin | E-Cadherin | |
| IL-27 | 0.835 (±0.097) | 1.180 (±0.024) | 1.233 (±0.108) | 1.148 (±0.121) |
| LPS + TNF | 1.037 (±0.068) | 0.737 (±0.070) | 0.939 (±0.017) | 0.737 (±0.070) |
| IL-27 + LPS + TNF | 0.774 (±0.097) | 1.182 (±0.020) | 1.095 (±0.075) | 1.152 (±0.070) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brice, D.P.; Murray, G.I.; Wilson, H.M.; Porter, R.J.; Berry, S.; Durum, S.K.; McLean, M.H. Interleukin-27 Regulates the Function of the Gastrointestinal Epithelial Barrier in a Human Tissue-Derived Organoid Model. Biology 2022, 11, 427. https://doi.org/10.3390/biology11030427
Brice DP, Murray GI, Wilson HM, Porter RJ, Berry S, Durum SK, McLean MH. Interleukin-27 Regulates the Function of the Gastrointestinal Epithelial Barrier in a Human Tissue-Derived Organoid Model. Biology. 2022; 11(3):427. https://doi.org/10.3390/biology11030427
Chicago/Turabian StyleBrice, Daniel P., Graeme I. Murray, Heather M. Wilson, Ross J. Porter, Susan Berry, Scott K. Durum, and Mairi H. McLean. 2022. "Interleukin-27 Regulates the Function of the Gastrointestinal Epithelial Barrier in a Human Tissue-Derived Organoid Model" Biology 11, no. 3: 427. https://doi.org/10.3390/biology11030427
APA StyleBrice, D. P., Murray, G. I., Wilson, H. M., Porter, R. J., Berry, S., Durum, S. K., & McLean, M. H. (2022). Interleukin-27 Regulates the Function of the Gastrointestinal Epithelial Barrier in a Human Tissue-Derived Organoid Model. Biology, 11(3), 427. https://doi.org/10.3390/biology11030427