Ocean Acidification Alleviates Dwarf Eelgrass (Zostera noltii) Lipid Landscape Remodeling under Warming Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Thin Layer Chromatography (TLC) Lipid Separation and Fatty Acid Analysis
2.3. Statistical Analysis
3. Results
3.1. Total Leaf Fatty Acid
3.2. Lipid Landscape Relative Composition
3.3. Galactolipid Fatty Acid Profile
3.4. Phospholipid Fatty Acid Profile
3.5. Neutral Lipid Fatty Acid Profile
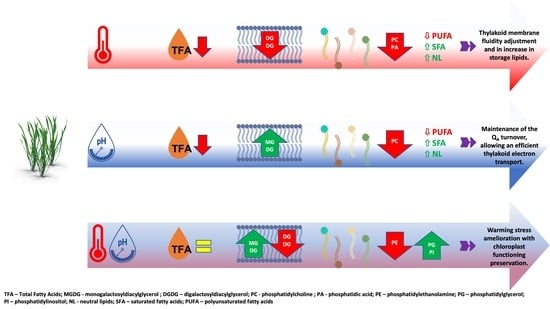
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duarte, B.; Matos, A.R.; Pedro, S.; Marques, J.C.; Adão, H.; Caçador, I. Dwarf Eelgrass (Zostera noltii) Leaf Fatty Acid Profile during a Natural Restoration Process: Physiological and Ecological Implications. Ecol. Indic. 2019, 106, 105452. [Google Scholar] [CrossRef]
- Duarte, B.; Matos, A.R.; Marques, J.C.; Caçador, I. Leaf Fatty Acid Remodeling in the Salt-Excreting Halophytic Grass Spartina Patens along a Salinity Gradient. Plant Physiol. Biochem. 2018, 124, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major Role of Marine Vegetation on the Oceanic Carbon Cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hogarth, P.J. The Biology of Mangroves and Seagrasses, 3rd ed.; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-871654-9. [Google Scholar]
- Marbà, N.; Krause-Jensen, D.; Alcoverro, T.; Birk, S.; Pedersen, A.; Neto, J.M.; Orfanidis, S.; Garmendia, J.M.; Muxika, I.; Borja, A.; et al. Diversity of European Seagrass Indicators: Patterns within and across Regions. Hydrobiologia 2013, 704, 265–278. [Google Scholar] [CrossRef]
- Duarte, B.; Martins, I.; Rosa, R.; Matos, A.R.; Roleda, M.Y.; Reusch, T.B.H.; Engelen, A.H.; Serrão, E.A.; Pearson, G.A.; Marques, J.C.; et al. Climate Change Impacts on Seagrass Meadows and Macroalgal Forests: An Integrative Perspective on Acclimation and Adaptation Potential. Front. Mar. Sci. 2018, 5, 190. [Google Scholar] [CrossRef] [Green Version]
- Fraser, M.W.; Kendrick, G.A.; Statton, J.; Hovey, R.K.; Zavala-Perez, A.; Walker, D.I. Extreme Climate Events Lower Resilience of Foundation Seagrass at Edge of Biogeographical Range. J. Ecol. 2014, 102, 1528–1536. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating Loss of Seagrasses across the Globe Threatens Coastal Ecosystems. Proc. Natl. Acad. Sci. 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [Green Version]
- Chefaoui, R.M.; Duarte, C.M.; Serrão, E.A. Dramatic Loss of Seagrass Habitat under Projected Climate Change in the Mediterranean Sea. Glob. Chang. Biol. 2018, 24, 4919–4928. [Google Scholar] [CrossRef]
- Short, F.T.; Koch, E.W.; Creed, J.C.; Magalhães, K.M.; Fernandez, E.; Gaeckle, J.L. SeagrassNet Monitoring across the Americas: Case Studies of Seagrass Decline. Mar. Ecol. 2006, 27, 277–289. [Google Scholar] [CrossRef]
- Cardoso, P.G.; Leston, S.; Grilo, T.F.; Bordalo, M.D.; Crespo, D.; Raffaelli, D.; Pardal, M.A. Implications of Nutrient Decline in the Seagrass Ecosystem Success. Mar. Pollut. Bull. 2010, 60, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Lefcheck, J.S.; Orth, R.J.; Dennison, W.C.; Wilcox, D.J.; Murphy, R.R.; Keisman, J.; Gurbisz, C.; Hannam, M.; Landry, J.B.; Moore, K.A.; et al. Long-Term Nutrient Reductions Lead to the Unprecedented Recovery of a Temperate Coastal Region. Proc. Natl. Acad. Sci. USA 2018, 115, 3658–3662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, C.M.; Agusti, S.; Barbier, E.; Britten, G.L.; Castilla, J.C.; Gattuso, J.-P.; Fulweiler, R.W.; Hughes, T.P.; Knowlton, N.; Lovelock, C.E.; et al. Rebuilding Marine Life. Nature 2020, 580, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Tomasko, D.; Alderson, M.; Burnes, R.; Hecker, J.; Leverone, J.; Raulerson, G.; Sherwood, E. Widespread Recovery of Seagrass Coverage in Southwest Florida (USA): Temporal and Spatial Trends and Management Actions Responsible for Success. Mar. Pollut. Bull. 2018, 135, 1128–1137. [Google Scholar] [CrossRef]
- de los Santos, C.B.; Krause-Jensen, D.; Alcoverro, T.; Marbà, N.; Duarte, C.M.; van Katwijk, M.M.; Pérez, M.; Romero, J.; Sánchez-Lizaso, J.L.; Roca, G.; et al. Recent Trend Reversal for Declining European Seagrass Meadows. Nat. Commun. 2019, 10, 3356. [Google Scholar] [CrossRef] [Green Version]
- Jordà, G.; Marbà, N.; Duarte, C.M. Mediterranean Seagrass Vulnerable to Regional Climate Warming. Nat. Clim. Chang. 2012, 2, 821–824. [Google Scholar] [CrossRef] [Green Version]
- Collier, C.J.; Ow, Y.X.; Langlois, L.; Uthicke, S.; Johansson, C.L.; O’Brien, K.R.; Hrebien, V.; Adams, M.P. Optimum Temperatures for Net Primary Productivity of Three Tropical Seagrass Species. Front. Plant Sci. 2017, 8, 1446. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Marín, F.; Brun, F.G.; Pedersen, M.F. Additive Response to Multiple Environmental Stressors in the Seagrass Zostera marina L. Limnol. Oceanogr. 2018, 63, 1528–1544. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Goldfarb, L.; Gomis, M.I.; Huang, M.; et al. IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Diaz-Almela, E.; Marbà, N.; Duarte, C.M. Consequences of Mediterranean Warming Events in Seagrass (Posidonia oceanica) Flowering Records. Glob. Chang. Biol. 2007, 13, 224–235. [Google Scholar] [CrossRef]
- Jueterbock, A.; Tyberghein, L.; Verbruggen, H.; Coyer, J.A.; Olsen, J.L.; Hoarau, G. Climate Change Impact on Seaweed Meadow Distribution in the North Atlantic Rocky Intertidal. Ecol. Evol. 2013, 3, 1356–1373. [Google Scholar] [CrossRef] [Green Version]
- Duarte, B.; Santos, D.; Silva, H.; Marques, J.C.; Caçador, I.; Sleimi, N. Light–Dark O2 Dynamics in Submerged Leaves of C3 and C4 Halophytes under Increased Dissolved CO2: Clues for Saltmarsh Response to Climate Change. AoB PLANTS 2014, 6, plu067. [Google Scholar] [CrossRef] [Green Version]
- Duarte, B.; Santos, D.; Silva, H.; Marques, J.C.; Caçador, I. Photochemical and Biophysical Feedbacks of C3 and C4 Mediterranean Halophytes to Atmospheric CO2 Enrichment Confirmed by Their Stable Isotope Signatures. Plant Physiol. Biochem. 2014, 80, 10–22. [Google Scholar] [CrossRef]
- Pérez-Romero, J.A.; Duarte, B.; Barcia-Piedras, J.-M.; Matos, A.R.; Redondo-Gómez, S.; Caçador, I.; Mateos-Naranjo, E. Investigating the Physiological Mechanisms Underlying Salicornia Ramosissima Response to Atmospheric CO2 Enrichment under Coexistence of Prolonged Soil Flooding and Saline Excess. Plant Physiol. Biochem. 2019, 135, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Romero, J.A.; Idaszkin, Y.L.; Duarte, B.; Baeta, A.; Marques, J.C.; Redondo-Gómez, S.; Caçador, I.; Mateos-Naranjo, E. Atmospheric CO2 Enrichment Effect on the Cu-Tolerance of the C4 Cordgrass Spartina densiflora. J. Plant Physiol. 2018, 220, 155–166. [Google Scholar] [CrossRef]
- Pérez-Romero, J.A.; Idaszkin, Y.L.; Barcia-Piedras, J.-M.; Duarte, B.; Redondo-Gómez, S.; Caçador, I.; Mateos-Naranjo, E. Disentangling the Effect of Atmospheric CO2 Enrichment on the Halophyte Salicornia Ramosissima J. Woods Physiological Performance under Optimal and Suboptimal Saline Conditions. Plant Physiol. Biochem. 2018, 127, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Bowes, G.; Ross, C.; Zhang, X.H. Climate Change and Ocean Acidification Effects on Seagrasses and Marine Macroalgae. Glob. Chang. Biol. 2013, 19, 103–132. [Google Scholar] [CrossRef]
- Duarte, B.; Caçador, I.; Matos, A.R. Lipid Landscape Remodelling in Sarcocornia Fruticosa Green and Red Physiotypes. Plant Physiol. Biochem. 2020, 157, 128–137. [Google Scholar] [CrossRef]
- Duarte, B.; Cabrita, M.T.; Gameiro, C.; Matos, A.R.; Godinho, R.; Marques, J.C.; Caçador, I. Disentangling the Photochemical Salinity Tolerance in Aster tripolium L.: Connecting Biophysical Traits with Changes in Fatty Acid Composition. Plant Biol. J. 2017, 19, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Feijão, E.; Franzitta, M.; Cabrita, M.T.; Caçador, I.; Duarte, B.; Gameiro, C.; Matos, A.R. Marine Heat Waves Alter Gene Expression of Key Enzymes of Membrane and Storage Lipids Metabolism in Phaeodactylum Tricornutum. Plant Physiol. Biochem. 2020, 156, 357–368. [Google Scholar] [CrossRef] [PubMed]
- den Hartog, C.; Kuo, J. Taxonomy and Biogeography of Seagrasses. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 1–23. [Google Scholar]
- Helmuth, B.; Mieszkowska, N.; Moore, P.; Hawkins, S.J. Living on the Edge of Two Changing Worlds: Forecasting the Responses of Rocky Intertidal Ecosystems to Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 373–404. [Google Scholar] [CrossRef] [Green Version]
- Massa, S.I.; Arnaud-Haond, S.; Pearson, G.A.; Serrão, E.A. Temperature Tolerance and Survival of Intertidal Populations of the Seagrass Zostera noltii (Hornemann) in Southern Europe (Ria Formosa, Portugal). Hydrobiologia 2009, 619, 195–201. [Google Scholar] [CrossRef]
- Short, F.T.; Neckles, H.A. The Effects of Global Climate Change on Seagrasses. Aquat. Bot. 1999, 63, 169–196. [Google Scholar] [CrossRef]
- Dubois, S.; Blanchet, H.; Garcia, A.; Massé, M.; Galois, R.; Grémare, A.; Charlier, K.; Guillou, G.; Richard, P.; Savoye, N. Trophic Resource Use by Macrozoobenthic Primary Consumers within a Semi-Enclosed Coastal Ecosystem: Stable Isotope and Fatty Acid Assessment. J. Sea Res. 2014, 88, 87–99. [Google Scholar] [CrossRef]
- van Ginneken, V.J.; Helsper, J.P.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated Fatty Acids in Various Macroalgal Species from North Atlantic and Tropical Seas. Lipids Health Dis. 2011, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Kainz, M.; Arts, M.T.; Mazumder, A. Essential Fatty Acids in the Planktonic Food Web and Their Ecological Role for Higher Trophic Levels. Limnol. Oceanogr. 2004, 49, 1784–1793. [Google Scholar] [CrossRef]
- Repolho, T.; Duarte, B.; Dionísio, G.; Paula, J.R.; Lopes, A.R.; Rosa, I.C.; Grilo, T.F.; Caçador, I.; Calado, R.; Rosa, R. Seagrass Ecophysiological Performance under Ocean Warming and Acidification. Sci. Rep. 2017, 7, 41443. [Google Scholar] [CrossRef]
- Matos, A.R.; Hourton-Cabassa, C.; Ciçek, D.; Rezé, N.; Arrabaça, J.D.; Zachowski, A.; Moreau, F. Alternative Oxidase Involvement in Cold Stress Response of Arabidopsis Thaliana Fad2 and FAD3+ Cell Suspensions Altered in Membrane Lipid Composition. Plant Cell Physiol. 2007, 48, 856–865. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Bahn, S.C.; Kang, Y.M.; Lee, K.H.; Kim, H.J.; Noh, E.K.; Palta, J.P.; Shin, J.S.; Ryu, S.B. Secretory Low Molecular Weight Phospholipase A2 Plays Important Roles in Cell Elongation and Shoot Gravitropism in Arabidopsis. Plant Cell 2003, 15, 1990–2002. [Google Scholar] [CrossRef] [Green Version]
- Esquível, M.G.; Matos, A.R.; Marques Silva, J. Rubisco Mutants of Chlamydomonas Reinhardtii Display Divergent Photosynthetic Parameters and Lipid Allocation. Appl. Microbiol. Biotechnol. 2017, 101, 5569–5580. [Google Scholar] [CrossRef]
- Matos, A.R.; Mendes, A.T.; Scotti-Campos, P.; Arrabaça, J.D. Study of the Effects of Salicylic Acid on Soybean Mitochondrial Lipids and Respiratory Properties Using the Alternative Oxidase as a Stress-Reporter Protein. Physiol. Plant. 2009, 137, 485–497. [Google Scholar] [CrossRef]
- Feijão, E.; Gameiro, C.; Franzitta, M.; Duarte, B.; Caçador, I.; Cabrita, M.T.; Matos, A.R. Heat Wave Impacts on the Model Diatom Phaeodactylum Tricornutum: Searching for Photochemical and Fatty Acid Biomarkers of Thermal Stress. Ecol. Indic. 2017, 95, 1026–1037. [Google Scholar] [CrossRef]
- Duarte, B.; Matos, A.R.; Caçador, I. Photobiological and Lipidic Responses Reveal the Drought Tolerance of Aster Tripolium Cultivated under Severe and Moderate Drought: Perspectives for Arid Agriculture in the Mediterranean. Plant Physiol. Biochem. 2020, 154, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Sebastiana, M.; Duarte, B.; Monteiro, F.; Malhó, R.; Caçador, I.; Matos, A.R. The Leaf Lipid Composition of Ectomycorrhizal Oak Plants Shows a Drought-Tolerance Signature. Plant Physiol. Biochem. 2019, 144, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Laureano, G.; Figueiredo, J.; Cavaco, A.R.; Duarte, B.; Caçador, I.; Malhó, R.; Sousa Silva, M.; Matos, A.R.; Figueiredo, A. The Interplay between Membrane Lipids and Phospholipase A Family Members in Grapevine Resistance against Plasmopara Viticola. Sci. Rep. 2018, 8, 14538. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; p. 192. [Google Scholar]
- Banzon, V.; Smith, T.M.; Chin, T.M.; Liu, C.; Hankins, W. A Long-Term Record of Blended Satellite and in Situ Sea-Surface Temperature for Climate Monitoring, Modeling and Environmental Studies. Earth Syst. Sci. Data 2016, 8, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Hobday, A.J.; Oliver, E.C.J.; Sen Gupta, A.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Holbrook, N.J.; Moore, P.J.; Thomsen, M.S.; Wernberg, T.; et al. Categorizing and Naming Marine Heatwaves. Oceanog 2018, 31, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, R.W.; Smit, A.J. HeatwaveR: A Central Algorithm for the Detection of Heatwaves and Cold-Spells. J. Open Source Softw. 2018, 3, 821. [Google Scholar] [CrossRef]
- Burger, F.A.; John, J.G.; Frölicher, T.L. Increase in Ocean Acidity Variability and Extremes under Increasing Atmospheric CO2. Biogeosciences 2020, 17, 4633–4662. [Google Scholar] [CrossRef]
- Cousins, A.B.; Adam, N.R.; Wall, G.W.; Kimball, B.A.; Pinter, P.J., Jr.; Ottman, M.J.; Leavitt, S.W.; Webber, A.N. Photosystem II Energy Use, Non-Photochemical Quenching and the Xanthophyll Cycle in Sorghumbicolor Grown under Drought and Free-Air CO2 Enrichment(FACE) Conditions. Plant Cell Environ. 2002, 25, 1551–1559. [Google Scholar] [CrossRef]
- Wall, G.W.; Brooks, T.J.; Adam, N.R.; Cousins, A.B.; Kimball, B.A.; Pinter, P.J., Jr.; LaMorte, R.L.; Triggs, J.; Ottman, M.J.; Leavitt, S.W.; et al. Elevated Atmospheric CO2 Improved Sorghum Plant Water Status by Ameliorating the Adverse Effects of Drought. New Phytol. 2001, 152, 231–248. [Google Scholar] [CrossRef]
- Hymus, G.J.; Ellsworth, D.S.; Baker, N.R.; Long, S.P. Does Free-Air Carbon Dioxide Enrichment Affect Photochemical Energy Use by Evergreen Trees in Different Seasons? A Chlorophyll Fluorescence Study of Mature Loblolly Pine1. Plant Physiol. 1999, 120, 1183–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. RISING ATMOSPHERIC CARBON DIOXIDE: Plants FACE the Future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, B.W.; Thornber, J.P. Assembly of the Light-Harvesting Complexes (LHCs) of Photosystem II (Monomeric LHC IIb Complexes Are Intermediates in the Formation of Oligomeric LHC IIb Complexes). Plant Physiol. 1994, 106, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yan, H.; Wang, K.; Kuang, T.; Zhang, J.; Gui, L.; An, X.; Chang, W. Crystal Structure of Spinach Major Light-Harvesting Complex at 2.72 Å Resolution. Nature 2004, 428, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, M.; Ruban, A.V.; Horton, P. The Functional Significance of the Monomeric and Trimeric States of the Photosystem II Light Harvesting Complexes. Biochemistry 2004, 43, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Seiwert, D.; Witt, H.; Janshoff, A.; Paulsen, H. The Non-Bilayer Lipid MGDG Stabilizes the Major Light-Harvesting Complex (LHCII) against Unfolding. Sci. Rep. 2017, 7, 5158. [Google Scholar] [CrossRef]
- Kern, J.; Guskov, A. Lipids in Photosystem II: Multifunctional Cofactors. J. Photochem. Photobiol. B Biol. 2011, 104, 19–34. [Google Scholar] [CrossRef]
- Duarte, B.; Goessling, J.W.; Marques, J.C.; Caçador, I. Ecophysiological Constraints of Aster Tripolium under Extreme Thermal Events Impacts: Merging Biophysical, Biochemical and Genetic Insights. Plant Physiol. Biochem. 2015, 97, 217–228. [Google Scholar] [CrossRef]
- Yu, B.; Benning, C. Anionic Lipids Are Required for Chloroplast Structure and Function in Arabidopsis. Plant J. 2003, 36, 762–770. [Google Scholar] [CrossRef]
- Carreiras, J.; Alberto Pérez-Romero, J.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Rita Matos, A.; Caçador, I.; Duarte, B. The Effect of Heavy Metal Contamination Pre-Conditioning in the Heat Stress Tolerance of Native and Invasive Mediterranean Halophytes. Ecol. Indic. 2020, 111, 106045. [Google Scholar] [CrossRef]
- Hobe, S.; Prytulla, S.; Kühlbrandt, W.; Paulsen, H. Trimerization and Crystallization of Reconstituted Light-Harvesting Chlorophyll a/b Complex. EMBO J. 1994, 13, 3423–3429. [Google Scholar] [CrossRef] [PubMed]
- Trémolières, A.; Roche, O.; Dubertret, G.; Guyon, D.; Garnier, J. Restoration of Thylakoid Appression by Δ3-Trans-Hexadecenoic Acid-Containing Phosphatidylglycerol in a Mutant of Chlamydomonas Reinhardtii. Relationships with the Regulation of Excitation Energy Distribution. BBA-Bioenerg. 1991, 1059, 286–292. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty Acid Unsaturation, Mobilization, and Regulation in the Response of Plants to Stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Mishkind, M. Phosphatidylethanolamine–In a Pinch PDZ Again: Establishing Cell Polarity. Trends Cell Biol. 2000, 10, 368. [Google Scholar] [CrossRef]
- Wan, C.; Kiessling, V.; Tamm, L.K. Coupling of Cholesterol-Rich Lipid Phases in Asymmetric Bilayers. Biochemistry 2008, 47, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Barg, R.; Yin, M.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated Fatty Acid Desaturation via Overexpression of Two Distinct ω-3 Desaturases Differentially Alters Tolerance to Various Abiotic Stresses in Transgenic Tobacco Cells and Plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef] [PubMed]
- König, S.; Ischebeck, T.; Lerche, J.; Stenzel, I.; Heilmann, I. Salt-Stress-Induced Association of Phosphatidylinositol 4,5-Bisphosphate with Clathrin-Coated Vesicles in Plants. Biochem. J. 2008, 415, 387–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Xu, W.; Burke, J.J.; Xin, Z. Role of Phosphatidic Acid in High Temperature Tolerance in Maize. Crop Sci. 2010, 50, 2506–2515. [Google Scholar] [CrossRef]
- Testerink, C.; Munnik, T. Molecular, Cellular, and Physiological Responses to Phosphatidic Acid Formation in Plants. J. Exp. Bot. 2011, 62, 2349–2361. [Google Scholar] [CrossRef] [Green Version]
- Ruggles, K.V.; Turkish, A.; Sturley, S.L. Making, Baking, and Breaking: The Synthesis, Storage, and Hydrolysis of Neutral Lipids. Annu. Rev. Nutr. 2013, 33, 413–451. [Google Scholar] [CrossRef]
- Franzitta, M.; Repolho, T.; Paula, J.R.; Caçador, I.; Matos, A.R.; Rosa, R.; Duarte, B. Dwarf Eelgrass (Zostera noltii) Fatty Acid Remodelling Induced by Climate Change. Estuar. Coast. Shelf Sci. 2021, 261, 107546. [Google Scholar] [CrossRef]
- Ferreira, D.; Figueiredo, J.; Laureano, G.; Machado, A.; Arrabaça, J.D.; Duarte, B.; Figueiredo, A.; Matos, A.R. Membrane Remodelling and Triacylglycerol Accumulation in Drought Stress Resistance: The Case Study of Soybean Phospholipases A. Plant Physiol. Biochem. 2021, 169, 9–21. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, B.; Repolho, T.; Paula, J.R.; Caçador, I.; Matos, A.R.; Rosa, R. Ocean Acidification Alleviates Dwarf Eelgrass (Zostera noltii) Lipid Landscape Remodeling under Warming Stress. Biology 2022, 11, 780. https://doi.org/10.3390/biology11050780
Duarte B, Repolho T, Paula JR, Caçador I, Matos AR, Rosa R. Ocean Acidification Alleviates Dwarf Eelgrass (Zostera noltii) Lipid Landscape Remodeling under Warming Stress. Biology. 2022; 11(5):780. https://doi.org/10.3390/biology11050780
Chicago/Turabian StyleDuarte, Bernardo, Tiago Repolho, José Ricardo Paula, Isabel Caçador, Ana Rita Matos, and Rui Rosa. 2022. "Ocean Acidification Alleviates Dwarf Eelgrass (Zostera noltii) Lipid Landscape Remodeling under Warming Stress" Biology 11, no. 5: 780. https://doi.org/10.3390/biology11050780
APA StyleDuarte, B., Repolho, T., Paula, J. R., Caçador, I., Matos, A. R., & Rosa, R. (2022). Ocean Acidification Alleviates Dwarf Eelgrass (Zostera noltii) Lipid Landscape Remodeling under Warming Stress. Biology, 11(5), 780. https://doi.org/10.3390/biology11050780