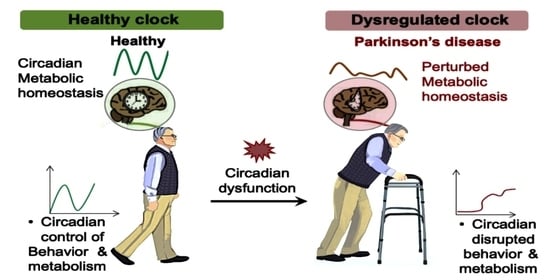
Metabolic Basis of Circadian Dysfunction in Parkinson’s Disease
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Transcription/Translation Feedback Loops (TTFL) Mechanisms in Mammals
1.2. Circadian Control of Cellular Metabolism
2. Circadian Dysfunction and PD
2.1. PD and Metabolism
2.2. Circadian Metabolism Changes Related to PD
2.2.1. Circadian System and Dopamine
2.2.2. Circadian Dysfunction of Serotonin Metabolism
2.2.3. Circadian Disruption of Energy Metabolism
2.2.4. Circadian Disruption of Hormone Metabolism
2.3. Neural Basis of Circadian Dysfunction in PD
2.4. Circadian Dysfunction in PD Patients
| S. No | Participants | Type of Circadian Markers | Measure of Circadian Markers | Results | Reference |
|---|---|---|---|---|---|
| 1 | 169 age- and sex-matched controls and 153 drug-naive patients with Parkinson’s disease (mean age, 66 years) | Excessive daytime sleepiness (EDS) | Epworth sleepiness scale (ESS) | At baseline, 12% of PD patients and 5% of controls had EDS; after 5 years on PD treatment, 23% of PD patients and 8% of controls still had EDS. | [29] |
| 2 | 20 PD patients and 15 controls of similar age (mean age 64 years) | Melatonin rhythm; EDS | 24 h repeated blood collection to measure plasma melatonin; ESS | When compared to controls, patients with PD had a four-fold lower 24 h AUC for circulating melatonin levels and a reduced melatonin rhythm amplitude (p = 0.0001); there was no discernible difference in DLMO. EDS was seen in 27% of controls and 60% of PD patients (p = 0.01). | [30] |
| 3 | 30 PD patients (mean age at diagnosis: 68 years) and 15 controls who were age and sex matched | Timing of sleep, peripheral clock gene expression, cortisol rhythm, melatonin rhythm, and EDS | 14-day actigraphy, ESS, and 24 h repeated blood samples for serum melatonin and cortisol | In comparison to controls, patients with PD had lower levels of circulating melatonin (p = 0.005), higher levels of cortisol (p = 0.0001), and altered Bmal1 expression (p = 0.004). Patients with PD also showed more fragmented motor activity over the course of 24 h and later sleep start time. | [48] |
| 4 | 28 age-matched controls and 29 patients with PD (mean age 642 years; 16 treated with medication, 13 not) | Timing of sleep, rhythm of melatonin, and phase angle of entrainment | salivary melatonin assay and 14-day actigraphy | The amount of melatonin secreted and the phase angle of entrainment were more than doubled (p = 0.001) in PD patients under dopaminergic therapy compared to controls, whereas there were no variations in sleep time or DLMO. | [28] |
| 5 | 12 PD patients (mean age, 62 years) and 11 age-matched controls were studied. | Timing of sleep and profile of core body temperature | 14-day actigraphy; 24 h ingestible capsule sensor used to record temperature profile | There was no change in sleep schedule that was statistically significant, although patients with PD had lower temperature mesors and lower nocturnal temperature amplitudes than controls. | [71] |
| 6 | 111 PD patients, average age 67.8 years | Blood pressure | A 2 -h ambulatory blood pressure check | PD patients showed a high burden of nocturnal hypertension and 71% of them did not typically see a drop in blood pressure at night. | [72] |
| 7 | 33 persons with Parkinson’s disease (age range: 52 to 72 years; mean age, SD) | Dim light melatonin | Melanopsin | Melanopsin-mediated post-illumination pupil response amplitudes were considerably decreased in PD (p = 0.0001) and linked with both nerve fiber layer thinning and poor sleep quality (r2 = 33 and 0.40, respectively, both p = 0.001). Higher subjective sleep ratings and earlier melatonin onset in people with Parkinson’s disease (PD) were both associated with significantly worse sleep quality (p = 0.05). Reaction of the outer retina to pupil lights the groups’ measurements, daily light exposure, and outer retinal thickness were comparable (p > 0.05). | [47] |
| 8 | 17 patients | Clock gene | BMAL1 PER | During the dark period, BMAL1’s expression pattern differed, whereas PER1’s did not. | [74] |
2.5. Circadian Dysfunction in the Animal Model of PD
2.5.1. Environmental Toxin Models
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Model
Rotenone Model
6-Hydroxydopamine (6-OHDA) Model
Manganese Model
2.5.2. Genetic Models
3. Management of PD through Circadian Specialized Medicine or Circadian Physiological Intervention
3.1. Light Intervention Can Manage PD
3.2. Physical Exercise Can Manage PD
3.3. Nutrient Intervention Can Manage PD
4. Challenges of Studying Circadian Metabolic Changes in PD
4.1. Selection of PD Model
4.2. Experimental Harvesting
4.3. Analysis of Altered Circadian Metabolome in PD
4.4. Identification of Altered Circadian Rhythmicity of Metabolites
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Husse, J.; Eichele, G.; Oster, H. Synchronization of the Mammalian Circadian Timing System: Light Can Control Peripheral Clocks Independently of the SCN Clock: Alternate Routes of Entrainment Optimize the Alignment of the Body’s Circadian Clock Network with External Time. BioEssays 2015, 37, 1119–1128. [Google Scholar] [CrossRef]
- Xiao, Y.; Yuan, Y.; Jimenez, M.; Soni, N.; Yadlapalli, S. Clock Proteins Regulate Spatiotemporal Organization of Clock Genes to Control Circadian Rhythms. Proc. Natl. Acad. Sci. USA 2021, 118, e2019756118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dai, M.; Wang, X.; Jiang, S.H.; Hu, L.P.; Zhang, X.L.; Zhang, Z.G. Signalling Entrains the Peripheral Circadian Clock. Cell. Signal. 2020, 69, 109433. [Google Scholar] [CrossRef] [PubMed]
- Feigl, B.; Dumpala, S.; Kerr, G.K.; Zele, A.J. Melanopsin Cell Dysfunction Is Involved in Sleep Disruption in Parkinson’s Disease. J. Park. Dis. 2020, 10, 1467–1476. [Google Scholar] [CrossRef]
- Monk, T.H.; Buysse, D.J.; Reynolds III, C.F.; Kupfer, D.J.; Houck, P.R. Circadian temperature Rhythms of Older People. Exp. Gerontol. 1995, 30, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Dyar, K.A.; Eckel-Mahan, K.L. Circadian Metabolomics in Time and Space. Front. Neurosci. 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.S.; Díaz, N.M.; D’Souza, S.; Buhr, E.D. The Molecular Clockwork of Mammalian Cells. Semin. Cell Dev. Biol. 2022, 126, 87–96. [Google Scholar] [CrossRef]
- Anna, G.; Kannan, N.N. Post-Transcriptional Modulators and Mediators of the Circadian Clock. Chronobiol. Int. 2021, 38, 1244–1261. [Google Scholar] [CrossRef]
- Park, J.W.; Roh, E.; Kang, G.M.; Gil, S.Y.; Kim, H.K.; Lee, C.H.; Jang, W.H.; Park, S.E.; Moon, S.Y.; Kim, S.J.; et al. Circulating Blood ENAMPT Drives the Circadian Rhythms in Locomotor Activity and Energy Expenditure. Nat. Commun. 2023, 14, 1994. [Google Scholar] [CrossRef]
- Yoshitane, H.; Asano, Y.; Sagami, A.; Sakai, S.; Suzuki, Y.; Okamura, H.; Iwasaki, W.; Ozaki, H.; Fukada, Y. Functional D-Box Sequences Reset the Circadian Clock and Drive MRNA Rhythms. Commun. Biol. 2019, 2, 300. [Google Scholar] [CrossRef]
- Duong, H.A.; Robles, M.S.; Knutti, D.; Weitz, C.J. A Molecular Mechanism for Circadian Clock Negative Feedback. Science 2011, 332, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Mauvoisin, D.; Wang, J.; Jouffe, C.; Martin, E.; Atger, F.; Waridel, P.; Quadroni, M.; Gachon, F.; Naef, F. Circadian Clock-Dependent and -Independent Rhythmic Proteomes Implement Distinct Diurnal Functions in Mouse Liver. Proc. Natl. Acad. Sci. USA 2014, 111, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ch, R.; Chevallier, O.; Elliott, C.T. Metabolomics Reveal Circadian Control of Cellular Metabolism. TrAC-Trends Anal. Chem. 2020, 130, 115986. [Google Scholar] [CrossRef]
- Bass, J. Circadian Topology of Metabolism. Nature 2012, 491, 348–356. [Google Scholar] [CrossRef]
- Dallmann, R.; Viola, A.U.; Tarokh, L.; Cajochen, C.; Brown, S.A. The Human Circadian Metabolome. Proc. Natl. Acad. Sci. USA 2012, 109, 2625–2629. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lozano Sinues, P.; Tarokh, L.; Li, X.; Kohler, M.; Brown, S.A.; Zenobi, R.; Dallmann, R. Circadian Variation of the Human Metabolome Captured by Real-Time Breath Analysis. PLoS ONE 2014, 9, 36–49. [Google Scholar] [CrossRef]
- Kasukawa, T.; Sugimoto, M.; Hida, A.; Minami, Y.; Mori, M.; Honma, S.; Honma, K.I.; Mishima, K.; Soga, T.; Ueda, H.R. Human Blood Metabolite Timetable Indicates Internal Body Time. Proc. Natl. Acad. Sci. USA 2012, 109, 15036–15041. [Google Scholar] [CrossRef]
- Ang, J.E.; Revell, V.; Mann, A.; Mäntele, S.; Otway, D.T.; Johnston, J.D.; Thumser, A.E.; Skene, D.J.; Raynaud, F. Identification of Human Plasma Metabolites Exhibiting Time-of-Day Variation Using an Untargeted Liquid Chromatographymass Spectrometry Metabolomic Approach. Chronobiol. Int. 2012, 29, 868–881. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Peek, C.B.; Affinati, A.H.; Ramsey, K.M.; Kuo, H.Y.; Yu, W.; Sena, L.A.; Ilkayeva, O.; Marcheva, B.; Kobayashi, Y.; Omura, C.; et al. Circadian Clock NAD+ Cycle Drives Mitochondrial Oxidative Metabolism in Mice. Science 2013, 342, 1243417. [Google Scholar] [CrossRef]
- Zwighaft, Z.; Aviram, R.; Shalev, M.; Rousso-Noori, L.; Kraut-Cohen, J.; Golik, M.; Brandis, A.; Reinke, H.; Aharoni, A.; Kahana, C.; et al. Circadian Clock Control by Polyamine Levels through a Mechanism That Declines with Age. Cell Metab. 2015, 22, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Orr, A.L.; Perevoshchikova, I.V.; Quinlan, C.L. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br. J. Dermatol. 2013, 169, 1–8. [Google Scholar] [CrossRef]
- Ch, R.; Rey, G.; Ray, S.; Jha, P.K.; Driscoll, P.C.; Dos Santos, M.S.; Malik, D.M.; Lach, R.; Weljie, A.M.; MacRae, J.I.; et al. Rhythmic Glucose Metabolism Regulates the Redox Circadian Clockwork in Human Red Blood Cells. Nat. Commun. 2021, 12, 377. [Google Scholar] [CrossRef]
- Jost, W.H.; Reichmann, H. “An Essay on the Shaking Palsy” 200 Years Old. J. Neural Transm. 2017, 124, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Coulson, E.J.; Rajnarayanan, R.; Oster, H.; Videnovic, A.; Rawashdeh, O. Sleep and Circadian Rhythms in Parkinson’s Disease and Preclinical Models. Mol. Neurodegener. 2022, 17, 1–21. [Google Scholar] [CrossRef]
- Fagotti, J.; Targa, A.D.S.; Rodrigues, L.S.; Noseda, A.C.D.; Dorieux, F.W.C.; Scarante, F.F.; Ilkiw, J.L.; Louzada, F.M.; Chowdhury, N.R.; Veen, D.R.; et al. Chronic Sleep Restriction in the Rotenone Parkinson’ s Disease Model in Rats Reveals Peripheral Early-Phase Biomarkers. Sci. Rep. 2019, 9, 1898. [Google Scholar] [CrossRef]
- Al-Bachari, S.; Naish, J.H.; Parker, G.J.M.; Emsley, H.C.A.; Parkes, L.M. Blood–Brain Barrier Leakage Is Increased in Parkinson’s Disease. Front. Physiol. 2020, 11, 593026. [Google Scholar] [CrossRef] [PubMed]
- Bolitho, S.J.; Naismith, S.L.; Rajaratnam, S.M.W.; Grunstein, R.R.; Hodges, J.R.; Terpening, Z.; Rogers, N.; Lewis, S.J.G. Disturbances in Melatonin Secretion and Circadian Sleep-Wake Regulation in Parkinson Disease. Sleep Med. 2014, 15, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Tholfsen, L.K.; Larsen, J.P.; Schulz, J.; Tysnes, O.B.; Gjerstad, M.D. Development of Excessive Daytime Sleepiness in Early Parkinson Disease. Neurology 2015, 85, 162–168. [Google Scholar] [CrossRef]
- Videnovic, A.; Noble, C.; Reid, K.J.; Peng, J.; Turek, F.W.; Marconi, A.; Rademaker, A.W.; Simuni, T.; Zadikoff, C.; Zee, P.C. Circadian Melatonin Rhythm and Excessive Daytime Sleepiness in Parkinson Disease. JAMA Neurol. 2014, 71, 463–469. [Google Scholar] [CrossRef]
- Roede, J.R.; Uppal, K.; Park, Y.; Lee, K.; Tran, V.; Walker, D.; Strobel, F.H.; Rhodes, S.L.; Ritz, B.; Jones, D.P. Serum Metabolomics of Slow vs. Rapid Motor Progression Parkinson’s Disease: A Pilot Study. PLoS ONE 2013, 8, e77629. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Santosh, W.; Kumar, S.; Christlet, H.T.T. Metabolic Profiling of Parkinson’s Disease: Evidence of Biomarker from Gene Expression Analysis and Rapid Neural Network Detection. J. Biomed. Sci. 2009, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Trupp, M.; Jonsson, P.; Ohrfelt, A.; Zetterberg, H.; Obudulu, O.; Malm, L.; Wuolikainen, A.; Linder, J.; Moritz, T.; Blennow, K.; et al. Metabolite and Peptide Levels in Plasma and CSF Differentiating Healthy Controls from Patients with Newly Diagnosed Parkinson’s Disease. J. Parkinsons Dis. 2014, 4, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Öhman, A.; Forsgren, L. NMR Metabonomics of Cerebrospinal Fluid Distinguishes between Parkinson’s Disease and Controls. Neurosci. Lett. 2015, 594, 36–39. [Google Scholar] [CrossRef]
- Kafka, M.S.; Benedito, M.A.; Roth, R.H.; Steele, L.K.; Wolfe, W.W.; Catravas, G.N. Circadian Rhythms in Catecholamine Metabolites and Cyclic Nucleotide Production. Chronobiol. Int. 1986, 3, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.K.; Ozburn, A.R.; McClung, C.A. Circadian Clock Genes: Effects on Dopamine, Reward and Addiction. Alcohol 2015, 49, 341–349. [Google Scholar] [CrossRef]
- Golombek, D.A.; Bussi, I.L.; Agostino, P.V. Minutes, Days and Years: Molecular Interactions among Different Scales of Biological Timing. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20120465. [Google Scholar] [CrossRef]
- Divito, C.B.; Steece-Collier, K.; Case, D.T.; Williams, S.P.G.; Stancati, J.A.; Zhi, L.; Rubio, M.E.; Sortwell, C.E.; Collier, T.J.; Sulzer, D.; et al. Loss of VGLUT3 Produces Circadian-Dependent Hyperdopaminergia and Ameliorates Motor Dysfunction and l-Dopa-Mediated Dyskinesias in a Model of Parkinson’s Disease. J. Neurosci. 2015, 35, 14983–14999. [Google Scholar] [CrossRef]
- Gillies, G.E.; Pienaar, I.S.; Vohra, S.; Qamhawi, Z. Sex Differences in Parkinson’s Disease. Front. Neuroendocrinol. 2014, 35, 370–384. [Google Scholar] [CrossRef]
- Mattam, U.; Jagota, A. Daily Rhythms of Serotonin Metabolism and the Expression of Clock Genes in Suprachiasmatic Nucleus of Rotenone-Induced Parkinson’s Disease Male Wistar Rat Model and Effect of Melatonin Administration. Biogerontology 2015, 16, 109–123. [Google Scholar] [CrossRef]
- Pacelli, C.; Rotundo, G.; Lecce, L.; Menga, M.; Bidollari, E.; Scrima, R.; Cela, O.; Piccoli, C.; Cocco, T.; Vescovi, A.L.; et al. Parkin Mutation Affects Clock Gene-Dependent Energy Metabolism. Int. J. Mol. Sci. 2019, 20, 2772. [Google Scholar] [CrossRef] [PubMed]
- Paulose, J.K.; Rucker, E.B.; Cassone, V.M. Toward the Beginning of Time: Circadian Rhythms in Metabolism Precede Rhythms in Clock Gene Expression in Mouse Embryonic Stem Cells. PLoS ONE 2012, 7, e49555. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Matsunaga, N.; Okazaki, H.; Kakimoto, K.; Kimura, Y.; Azuma, H.; Ikeda, E.; Shiba, T.; Yamato, M.; Yamada, K.I.; et al. A Disruption Mechanism of the Molecular Clock in a MPTP Mouse Model of Parkinson’s Disease. NeuroMolecular Med. 2013, 15, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Wang, F.; Hu, L.F.; Liu, C.F. A New Perspective for Parkinson’s Disease: Circadian Rhythm. Neurosci. Bull. 2017, 33, 62–72. [Google Scholar] [CrossRef]
- Breen, D.P.; Nombela, C.; Vuono, R.; Jones, P.S.; Fisher, K.; Burn, D.J.; Brooks, D.J.; Reddy, A.B.; Rowe, J.B.; Barker, R.A. Hypothalamic Volume Loss Is Associated with Reduced Melatonin Output in Parkinson’s Disease. Mov. Disord. 2016, 31, 1062–1066. [Google Scholar] [CrossRef]
- Hartmann, A.; Veldhuis, J.D.; Deuschle, M.; Standhardt, H.; Heuser, I. Twenty-Four Hour Cortisol Release Profiles in Patients with Alzheimer’s and Parkinson’s Disease Compared to Normal Controls: Ultradian Secretory Pulsatility and Diurnal Variation. Neurobiol. Aging 1997, 18, 285–289. [Google Scholar] [CrossRef]
- Breen, D.P.; Vuono, R.; Nawarathna, U.; Fisher, K.; Shneerson, J.M.; Reddy, A.B.; Barker, R.A. Sleep and Circadian Rhythm Regulation in Early Parkinson Disease. JAMA Neurol. 2014, 71, 589–595. [Google Scholar] [CrossRef]
- Weaver, D.R. The Suprachiasmatic Nucleus: A 25-Year Retrospective. J. Biol. Rhythm. 1998, 13, 100–112. [Google Scholar] [CrossRef]
- Saeb-Parsy, K.; Lombardelli, S.; Khan, F.Z.; McDowall, K.; Au-Yong, I.T.H.; Dyball, R.E.J. Neural Connections of Hypothalamic Neuroendocrine Nuclei in the Rat. J. Neuroendocrinol. 2000, 12, 635–648. [Google Scholar] [CrossRef]
- Yi, C.X.; Van Der Vliet, J.; Dai, J.; Yin, G.; Ru, L.; Buijs, R.M. Ventromedial Arcuate Nucleus Communicates Peripheral Metabolic Information to the Suprachiasmatic Nucleus. Endocrinology 2006, 147, 283–294. [Google Scholar] [CrossRef]
- Berk, M.L.; Finkelstein, J.A. An Autoradiographic Determination of the Efferent Projections of the Suprachiasmatic Nucleus of the Hypothalamus. Brain Res. 1981, 226, 1–13. [Google Scholar] [CrossRef]
- Vrang, N.; Mikkelsen, J.D.; Larsen, P.J. Direct Link from the Suprachiasmatic Nucleus to Hypothalamic Neurons Projecting to the Spinal Cord: A Combined Tracing Study Using Cholera Toxin Subunit B and Phaseolus Vulgaris-Leucoagglutinin. Brain Res. Bull. 1997, 44, 671–680. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Palm, I.F.; La Fleur, S.E.; Scheer, F.A.J.L.; Perreau-Lenz, S.; Ruiter, M.; Kreier, F.; Cailotto, C.; Buijs, R.M. SCN Outputs and the Hypothalamic Balance of Life. J. Biol. Rhythms 2006, 21, 458–469. [Google Scholar] [CrossRef]
- Luo, A.H.; Aston-Jones, G. Circuit Projection from Suprachiasmatic Nucleus to Ventral Tegmental Area: A Novel Circadian Output Pathway. Eur. J. Neurosci. 2009, 29, 748–760. [Google Scholar] [CrossRef]
- Chemelli, R.M.; Willie, J.T.; Sinton, C.M.; Elmquist, J.K.; Scammell, T.; Lee, C.; Richardson, J.A.; Clay Williams, S.; Xiong, Y.; Kisanuki, Y.; et al. Narcolepsy in Orexin Knockout Mice: Molecular Genetics of Sleep Regulation. Cell 1999, 98, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Becker-Krail, D.D.; Walker, W.H.; Nelson, R.J. The Ventral Tegmental Area and Nucleus Accumbens as Circadian Oscillators: Implications for Drug Abuse and Substance Use Disorders. Front. Physiol. 2022, 13, 886704. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.A.; Sidiropoulou, K.; Vitaterna, M.; Takahashi, J.S.; White, F.J.; Cooper, D.C.; Nestler, E.J. Regulation of Dopaminergic Transmission and Cocaine Reward by the Clock Gene. Proc. Natl. Acad. Sci. USA 2005, 102, 9377–9381. [Google Scholar] [CrossRef] [PubMed]
- Ferris, M.J.; España, R.A.; Locke, J.L.; Konstantopoulos, J.K.; Rose, J.H.; Chen, R.; Jones, S.R. Dopamine Transporters Govern Diurnal Variation in Extracellular Dopamine Tone. Proc. Natl. Acad. Sci. USA 2014, 111, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Hampp, G.; Ripperger, J.A.; Houben, T.; Schmutz, I.; Blex, C.; Perreau-Lenz, S.; Brunk, I.; Spanagel, R.; Ahnert-Hilger, G.; Meijer, J.H.; et al. Regulation of Monoamine Oxidase A by Circadian-Clock Components Implies Clock Influence on Mood. Curr. Biol. 2008, 18, 678–683. [Google Scholar] [CrossRef]
- Kim, J.; Jang, S.; Choe, H.K.; Chung, S.; Son, G.H.; Kim, K. Implications of Circadian Rhythm in Dopamine and Mood Regulation. Mol. Cells 2017, 40, 450–456. [Google Scholar] [CrossRef]
- Abreu, T.M.; Monteiro, V.S.; Martins, A.B.S.; Teles, F.B.; da Conceição Rivanor, R.L.; Mota, É.F.; Macedo, D.S.; de Vasconcelos, S.M.M.; Júnior, J.E.R.H.; Benevides, N.M.B. Involvement of the Dopaminergic System in the Antidepressant-like Effect of the Lectin Isolated from the Red Marine Alga Solieria Filiformis in Mice. Int. J. Biol. Macromol. 2018, 111, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Ulane, C.M.; Burke, R.E. Clinical Progression in Parkinson Disease and the Neurobiology of Axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A. Cholinergic and Noradrenergic Modulation of Thalamocortical Processing. Trends Neurosci. 1989, 12, 215–221. [Google Scholar] [CrossRef]
- Granados-Fuentes, D.; Prolo, L.M.; Abraham, U.; Herzog, E.D. The Suprachiasmatic Nucleus Entrains, but Does Not Sustain, Circadian Rhythmicity in the Olfactory Bulb. J. Neurosci. 2004, 24, 615–619. [Google Scholar] [CrossRef]
- Abe, M.; Herzog, E.D.; Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Circadian Rhythms in Isolated Brain Regions. J. Neurosci. 2002, 22, 350–356. [Google Scholar] [CrossRef]
- Guilding, C.; Piggins, H.D. Challenging the Omnipotence of the Suprachiasmatic Timekeeper: Are Circadian Oscillators Present throughout the Mammalian Brain? Eur. J. Neurosci. 2007, 25, 3195–3216. [Google Scholar] [CrossRef]
- Feillet, C.A.; Mendoza, J.; Albrecht, U.; Pévet, P.; Challet, E. Forebrain Oscillators Ticking with Different Clock Hands. Mol. Cell. Neurosci. 2008, 37, 209–221. [Google Scholar] [CrossRef]
- Nestler, E.J.; Carlezon, W.A. The Mesolimbic Dopamine Reward Circuit in Depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Myung, J.; Schmal, C.; Hong, S.; Tsukizawa, Y.; Rose, P.; Zhang, Y.; Holtzman, M.J.; De Schutter, E.; Herzel, H.; Bordyugov, G.; et al. The Choroid Plexus Is an Important Circadian Clock Component. Nat. Commun. 2018, 9, 1062. [Google Scholar] [CrossRef]
- Barone, P.; Antonini, A.; Colosimo, C.; Marconi, R.; Morgante, L.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, G.; Ceravolo, R.; et al. The PRIAMO Study: A Multicenter Assessment of Nonmotor Symptoms and Their Impact on Quality of Life in Parkinson’s Disease. Mov. Disord. 2009, 24, 1641–1649. [Google Scholar] [CrossRef]
- Zhong, G.; Bolitho, S.; Grunstein, R.; Naismith, S.L.; Lewis, S.J.G. The Relationship between Thermoregulation and REM Sleep Behaviour Disorder in Parkinson’s Disease. PLoS ONE 2013, 8, e72661. [Google Scholar] [CrossRef] [PubMed]
- Berganzo, K.; Díez-Arrola, B.; Tijero, B.; Somme, J.; Lezcano, E.; Llorens, V.; Ugarriza, I.; Ciordia, R.; Gómez-Esteban, J.C.; Zarranz, J.J. Nocturnal Hypertension and Dysautonomia in Patients with Parkinson’s Disease: Are They Related? J. Neurol. 2013, 260, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Matsumura, R.; Tokuda, I.T.; Yoshikawa, T.; Shigeyoshi, Y.; Node, K.; Sakoda, S.; Akashi, M. Bright Light Improves Sleep in Patients with Parkinson’s Disease: Possible Role of Circadian Restoration. Sci. Rep. 2020, 10, 7982. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, S.; Sothern, R.B.; Xu, S.; Chan, P. Expression of Clock Genes Per1 and Bmal1 in Total Leukocytes in Health and Parkinson’s Disease. Eur. J. Neurol. 2010, 17, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Wei, S.Z.; Huang, G.D.; Liu, L.B.; Gu, C.; Shen, Y.; Wang, X.H.; Xia, S.T.; Xie, A.M.; Hu, L.F.; et al. BMAL1 Regulation of Microglia-Mediated Neuroinflammation in MPTP-Induced Parkinson’s Disease Mouse Model. FASEB J. 2020, 34, 6570–6581. [Google Scholar] [CrossRef]
- Lauretti, E.; Di Meco, A.; Merali, S.; Praticò, D. Circadian Rhythm Dysfunction: A Novel Environmental Risk Factor for Parkinson’s Disease. Mol. Psychiatry 2017, 22, 280–286. [Google Scholar] [CrossRef]
- Kim, J.; Park, I.; Jang, S.; Choi, M.; Kim, D.; Sun, W.; Choe, Y.; Choi, J.W.; Moon, C.; Park, S.H.; et al. Pharmacological Rescue with SR8278, a Circadian Nuclear Receptor REV-ERBA α Antagonist as a Therapy for Mood Disorders in Parkinson’s Disease. Neurotherapeutics 2022, 19, 592–607. [Google Scholar] [CrossRef]
- Franke, S.K.; van Kesteren, R.E.; Wubben, J.A.M.; Hofman, S.; Paliukhovich, I.; van der Schors, R.C.; van Nierop, P.; Smit, A.B.; Philippens, I.H.C.H.M. Progression and Recovery of Parkinsonism in a Chronic Progressive MPTP-Induction Model in the Marmoset without Persistent Molecular and Cellular Damage. Neuroscience 2016, 312, 247–259. [Google Scholar] [CrossRef]
- Li, H.; Song, S.; Wang, Y.; Huang, C.; Zhang, F.; Liu, J.; Hong, J. Low-Grade Inflammation Aggravates Rotenone Neurotoxicity and Disrupts Circadian Clock Gene Expression in Rats. Neurotox Res. 2019, 35, 421–431. [Google Scholar] [CrossRef]
- Jayapalan, J.J.; Subramanian, P.; Kani, A.; Hiji, J.; Najjar, S.G.; Rahman, P.S.A.; Hashim, O.H. Hesperidin Modulates the Rhythmic Proteomic Profiling in Drosophila Melanogaster under Oxidative Stress. Arch. Insect Biochem. Physiol. 2020, 105, e21738. [Google Scholar] [CrossRef]
- Gravotta, L.; Gavrila, A.M.; Hood, S.; Amir, S. Global Depletion of Dopamine Using Intracerebroventricular 6-Hydroxydopamine Injection Disrupts Normal Circadian Wheel-Running Patterns and PERIOD2 Expression in the Rat Forebrain. J. Mol. Neurosci. 2011, 45, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Fagundes, C.T.; Yang, G.; Palsson-McDermott, E.M.; Wochal, P.; McGettrick, A.F.; Foley, N.H.; Early, J.O.; Chen, L.; Zhang, H.; et al. Circadian Control of Innate Immunity in Macrophages by MiR-155 Targeting Bmal1. Proc. Natl. Acad. Sci. USA 2015, 112, 7231–7236. [Google Scholar] [CrossRef] [PubMed]
- Boulamery, A.; Simon, N.; Vidal, J.; Bruguerolle, B. Effects of L-Dopa on Circadian Rhythms of 6-Ohda Striatal Lesioned Rats: A Radiotelemetric Study. Chronobiol. Int. 2010, 27, 251–264. [Google Scholar] [CrossRef]
- Weng, H.; Song, W.; Fu, K.; Guan, Y.; Cai, G.; Huang, E.; Chen, X.; Zou, H.; Ye, Q. Proteomic Profiling Reveals the Potential Mechanisms and Regulatory Targets of Sirtuin 4 in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Mouse Model. Front. Neurosci. 2023, 16, 1035444. [Google Scholar] [CrossRef]
- Li, H.; Fan, X.; Luo, Y.; Song, S.; Liu, J.; Fan, Q. Repeated Manganese Administration Produced Abnormal Expression of Circadian Clock Genes in the Hypothalamus and Liver of Rats. Neurotoxicology 2017, 62, 39–45. [Google Scholar] [CrossRef]
- Valadas, J.S.; Esposito, G.; Vandekerkhove, D.; Miskiewicz, K.; Deaulmerie, L.; Raitano, S.; Seibler, P.; Klein, C.; Verstreken, P. ER Lipid Defects in Neuropeptidergic Neurons Impair Sleep Patterns in Parkinson’s Disease. Neuron 2018, 98, 1155–1169.e6. [Google Scholar] [CrossRef] [PubMed]
- Fifel, K.; Cooper, H.M. Loss of Dopamine Disrupts Circadian Rhythms in a Mouse Model of Parkinson’s Disease. Neurobiol. Dis. 2014, 71, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Lax, P.; Esquiva, G.; Esteve-Rudd, J.; Otalora, B.B.; Madrid, J.A.; Cuenca, N. Circadian Dysfunction in a Rotenone-Induced Parkinsonian Rodent Model. Chronobiol. Int. 2012, 29, 147–156. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, D.; Liu, W.; Li, S.; Chen, J.; Shen, Y.; Wang, F.; Hu, L.F.; Liu, C.F. Disruption of the Circadian Clock Alters Antioxidative Defense via the SIRT1-BMAL1 Pathway in 6-OHDA-Induced Models of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2018, 2018, 4854732. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, Y.L.; Liu, W.W.; Lyu, D.J.; Wang, F.; Mao, C.J.; Yang, Y.P.; Hu, L.F.; Liu, C.F. Long-Term Levodopa Treatment Accelerates the Circadian Rhythm Dysfunction in a 6-Hydroxydopamine Rat Model of Parkinson’s Disease. Chin. Med. J. 2017, 130, 1085–1092. [Google Scholar] [CrossRef]
- Hood, S.; Cassidy, P.; Cossette, M.P.; Weigl, Y.; Verwey, M.; Robinson, B.; Stewart, J.; Amir, S. Endogenous Dopamine Regulates the Rhythm of Expression of the Clock Protein PER2 in the Rat Dorsal Striatum via Daily Activation of D2 Dopamine Receptors. J. Neurosci. 2010, 30, 14046–14058. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N. Effects of Kamikihito and Unkei-to on Sleep Behavior of Wild Type and Parkinson Model in Drosophila. Front. Psychiatry 2017, 8, 2–8. [Google Scholar] [CrossRef]
- Peters, S.T.; Fahrenkopf, A.; Choquette, J.M.; Vermilyea, S.C.; Lee, M.K.; Vossel, K. Ablating Tau Reduces Hyperexcitability and Moderates Electroencephalographic Slowing in Transgenic Mice Expressing A53T Human α-Synuclein. Front. Neurol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Loh, D.H.; Truong, D.; Wu, Y.; Colwell, C.S. Circadian Dysfunction in a Mouse Model of Parkinson’s Disease. Exp. Neurol. 2011, 232, 66–75. [Google Scholar] [CrossRef]
- Doktór, B.; Damulewicz, M.; Pyza, E. Effects of MUL1 and PARKIN on the Circadian Clock, Brain and Behaviour in Drosophila Parkinson’s Disease Models. BMC Neurosci. 2019, 20, 24. [Google Scholar] [CrossRef]
- Videnovic, A.; Lazar, A.S.; Barker, R.A.; Overeem, S. “The Clocks That Time Us”-Circadian Rhythms in Neurodegenerative Disorders. Nat. Rev. Neurol. 2014, 10, 683–693. [Google Scholar] [CrossRef]
- Witkovsky, P. Dopamine and Retinal Function. Doc. Ophthalmol. 2004, 108, 17–39. [Google Scholar] [CrossRef]
- Paus, S.; Schmitz-Hübsch, T.; Wüllner, U.; Vogel, A.; Klockgether, T.; Abele, M. Bright Light Therapy in Parkinson’s Disease: A Pilot Study. Mov. Disord. 2007, 22, 1495–1498. [Google Scholar] [CrossRef]
- Videnovic, A.; Golombek, D. Circadian Dysregulation in Parkinson’s Disease. Neurobiol. Sleep Circadian Rhythm. 2017, 2, 53–58. [Google Scholar] [CrossRef]
- Willis, G.L.; Moore, C.; Armstrong, S.M. A Historical Justification for and Retrospective Analysis of the Systematic Application of Light Therapy in Parkinson’s Disease. Rev. Neurosci. 2012, 23, 199–226. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Hashimoto, S.; Masubuchi, S.; Natsubori, A.; Nishide, S.Y.; Honma, S.; Honma, K.I. Differential Regulation of Circadian Melatonin Rhythm and Sleep-Wake Cycle by Bright Lights and Nonphotic Time Cues in Humans. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2014, 307, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, Y.; Nakao, R.; Oishi, K. Free Access to a Running-Wheel Advances the Phase of Behavioral and Physiological Circadian Rhythms and Peripheral Molecular Clocks in Mice. PLoS ONE 2015, 10, e0116476. [Google Scholar] [CrossRef]
- Bordet, R.; Devos, D.; Brique, S.; Touitou, Y.; Guieu, J.D.; Libersa, C.; Destée, A. Study of Circadian Melatonin Secretion Pattern at Different Stages of Parkinson’s Disease. Clin. Neuropharmacol. 2003, 26, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Fertl, E.; Auff, E.; Doppelbauer, A.; Waldhauser, F. Circadian Secretion Pattern of Melatonin in de Novo Parkinsonian Patients: Evidence for Phase-Shifting Properties of l-Dopa. J. Neural Transm. -Park. Dis. Dement. Sect. 1993, 5, 227–234. [Google Scholar] [CrossRef]
- Kin, K.; Yasuhara, T.; Kameda, M.; Date, I. Animal Models for Parkinson’s Disease Research: Trends in the 2000s. Int. J. Mol. Sci. 2019, 20, 5402. [Google Scholar] [CrossRef]
- De Lazzari, F.; Bisaglia, M.; Zordan, M.A.; Sandrelli, F. Circadian Rhythm Abnormalities in Parkinson’s Disease from Humans to Flies and Back. Int. J. Mol. Sci. 2018, 19, 3911. [Google Scholar] [CrossRef]
- Hobson, D.E.; Lang, A.E.; Wayne Martin, W.R.; Razmy, A.; Rivest, J.; Fleming, J. Excessive Daytime Sleepiness and Sudden-Onset Sleep in Parkinson Disease: A Survey by the Canadian Movement Disorders Group. JAMA 2002, 287, 455–463. [Google Scholar] [CrossRef]
- Hughes, M.E.; Abruzzi, K.C.; Allada, R.; Anafi, R.; Arpat, A.B.; Asher, G.; Baldi, P.; de Bekker, C.; Bell-Pedersen, D.; Blau, J.; et al. Guidelines for Genome-Scale Analysis of Biological Rhythms. J. Biol. Rhythms 2017, 32, 380–393. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern Analytical Techniques in Metabolomics Analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef]
- Taylor, M.J.; Lukowski, J.K.; Anderton, C.R. Spatially Resolved Mass Spectrometry at the Single Cell: Recent Innovations in Proteomics and Metabolomics. J. Am. Soc. Mass Spectrom. 2021, 32, 872–894. [Google Scholar] [CrossRef]
- Tang, D.-Q.; Zou, L.; Yin, X.-X.; Ong, C.N. HILIC-MS for metabolomics: An attractive and complementary approach to RPLC-MS. Mass Spectrom. Rev. 2016, 35, 574–600. [Google Scholar] [CrossRef] [PubMed]
- Dyar, K.A.; Lutter, D.; Artati, A.; Ceglia, N.J.; Liu, Y.; Armenta, D.; Jastroch, M.; Schneider, S.; de Mateo, S.; Cervantes, M.; et al. Atlas of Circadian Metabolism Reveals System-Wide Coordination and Communication between Clocks. Cell 2018, 174, 1571–1585.e11. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Jiang, Z.; Chen, Y.; Chen, L.; Sancar, A.; Jiang, Y. Genome-Wide Circadian Rhythm Detection Methods: Systematic Evaluations and Practical Guidelines. Brief. Bioinform. 2021, 22, bbaa135. [Google Scholar] [CrossRef] [PubMed]
- Deckard, A.; Anafi, R.C.; Hogenesch, J.B.; Haase, S.B.; Harer, J.; Valencia, A. Design and Analysis of Large-Scale Biological Rhythm Studies: A Comparison of Algorithms for Detecting Periodic Signals in Biological Data. Bioinformatics 2013, 29, 3174–3180. [Google Scholar] [CrossRef] [PubMed]





| S. No | PD Model | Animal | Experimental Method | Circadian Rhythms Changes | Ref. |
|---|---|---|---|---|---|
| 1. | MPTP model | Mice | qRT-PCR, WB, IHC | Mice lacking the BMAL1 gene treated with MPTP show a 60% reduction in tyrosine hydroxylase (TH) protein levels. | [75] |
| 2. | MPTP Model | Mice | IHC, Behavior analysis | Circadian disruption causes greater loss of TH cell content and intense neuroinflammation. | [76] |
| 3. | MPTP Model | Mice | Bioluminescence, RT-qPCR | Activation of AMPK results in circadian disruption, according to Bmal1, Cry1, and Rev-ErbA α a. | [44] |
| 4. | MPTP Model | Mice | Behavior analysis and IHC | Lengthened free-running period. | [77] |
| 5. | MPTP Model | Non-human Primates | IHC, Proteomics | Alteration in circadian rhythms (not significantly). | [78] |
| 6. | Rotenone Model | Rat | Chronic Sleep Restriction | Affected a number of behavioral (reversal of locomotor activity impairment; cognitive impairment; delay of rest-activity rhythm) and metabolic (branched-chain amino acids, tryptophan pathway, phenylalanine, and lipoproteins, pointing to mitochondrial impairment) measures. | [26] |
| 7. | Rotenone Model | Rats | Substantia nigra RT-qPCR, WB | Bmal1, Clock, NPAS2, Per 1 and 2, Rev-ErbA α a, and DBP. In RIPD rats, chronic low-grade neuroinflammation worsens circadian disruption. | [79] |
| 8. | Rotenone Model | Rat | Behavior analysis | Reduced rhythm amplitudes and increased fragmentation in rhythm. | [92] |
| 9. | Rotenone Model | Rat | qRT-PCR, WB, IHC | Lowered rhythm amplitudes, altered expression of clock genes, and increased rhythm fragmentation. | [41] |
| 10. | 6-OHDA Model | Rat | IHC, Constant dark | Activity decline and circadian activity rhythm interruption. | [81] |
| 11. | 6-OHDA model | Rat | Dopamine and levodopa measurement | Loss of circadian rhythmicity or changes. | [83] |
| 12. | 6-OHDA Model | Rat and neuroblastoma cells | Striatum for RT-qPCR; WB | Through SIRT1-dependent BMAL1 pathways, dysfunction of the circadian clock contributes to an aberrant antioxidant response in PD. | [93] |
| 13. | 6-OHDA Model | Rat | Striatum, SCN Plasma RT-qPCR, ELISA, HPLC | Bmal1 decrease, peak of Per2 delayed, cortisol secretion increased, and melatonin level decreased after levodopa treatment. | [85] |
| 14. | 6-OHDA Model | Rat | Immunostaining RT-PCR, HPLC | The frequency of dopaminergic activation of D2 DA receptors determines the rhythm of PER2 expression in the dorsal striatum. | [94] |
| 15. | Mn2+ | Rat | Hypothalamus, RT-qPCR, IHC | A few examples are an increase in Nr1d1 and DBP and a decrease in Bmal1, Clock, NPAS2, Cry1, Per1, and Per2. | [86] |
| 16. | A30P | Drosophila | Behavior | Total amount of sleep is significantly reduced. | [95] |
| 17. | PARK and PINK1 mutant | Drosophila melanogaster | RT-qPCR, WB IHC, LIPID | Greater sleep fragmentation and lower circadian power. Phosphatidylserine from the endoplasmic reticulum (ER) and disrupts the production of neuropeptide-containing vesicles. | [90] |
| 18. | Mutant -SYN (A53T) | Mouse | EEG | Decreased total sleep time and NREM sleep. | [87] |
| 19. | ASO Transgenic | Mice | IHC | The SCN of ASO mice do not exhibit changed Per2 expression, and PD is characterized by diminished circadian output. | [88] |
| 20. | Mul 1A6 and Park1 mutants | Drosophila | RT-qPCR, IHC, WB | Per, Tim, and Clock’s typical circadian rhythmic expression during the day is interfered with by Mul 1 and Park mutations decreased ATG5. | [96] |
| 21. | Mitopark | mouse | Increased sleep latency. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathor, P.; Ch, R. Metabolic Basis of Circadian Dysfunction in Parkinson’s Disease. Biology 2023, 12, 1294. https://doi.org/10.3390/biology12101294
Rathor P, Ch R. Metabolic Basis of Circadian Dysfunction in Parkinson’s Disease. Biology. 2023; 12(10):1294. https://doi.org/10.3390/biology12101294
Chicago/Turabian StyleRathor, Priya, and Ratnasekhar Ch. 2023. "Metabolic Basis of Circadian Dysfunction in Parkinson’s Disease" Biology 12, no. 10: 1294. https://doi.org/10.3390/biology12101294
APA StyleRathor, P., & Ch, R. (2023). Metabolic Basis of Circadian Dysfunction in Parkinson’s Disease. Biology, 12(10), 1294. https://doi.org/10.3390/biology12101294