Improving Dietary Zinc Bioavailability Using New Food Fortification Approaches: A Promising Tool to Boost Immunity in the Light of COVID-19
Abstract
:Simple Summary
Abstract
1. Introduction
2. Overview of the Biological and Physiological Functions of Zinc
3. Zinc Homeostasis and Bioavailability
3.1. Zinc Homeostasis in the Body
3.2. Cellular Mechanism of Zinc Homeostasis
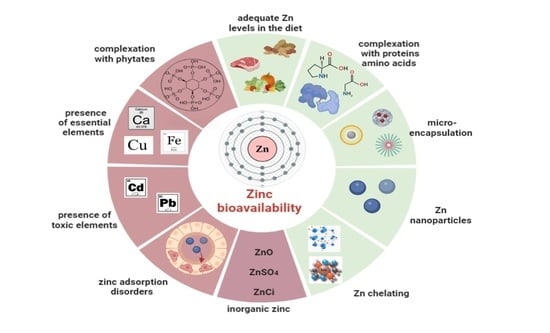
3.3. Zinc Bioavailability
4. Zinc as an Immunomodulatory Element and Its Implications for COVID-19
4.1. Zinc and Immunity
4.2. Zinc and COVID-19

5. Zinc and Food Products
5.1. Zinc Content in Food Product
5.2. Fortification/Biofortification of Food with Zinc
6. Zinc Encapsulation and Chelation as Novel Approaches to Increase Zinc Bioavailability in Food Fortification/Biofortification
6.1. Chelating Zinc with a Biological Compound
- Zinc-chelating polysaccharides
- Zinc-chelating peptides
| Zn Chelator Type | Zn Chelator Biological Source/Zn Precursors Used | Effect of the Zn Chelating Complex on Zn Bioavailability and Bioactivity | References |
|---|---|---|---|
| Polysaccharide (Ps) | Ginger (Zingiber officinale Roscoe) peel powder; pumpkin skin (Cucurbita moschata)/ZnSO4 | Ps–Zn complex could be used as a safe and effective form of Zn supplementation to prevent inflammatory reaction induced by copper sulfate (CuSO4) in zebrafish. | [85] |
| Prepared Athelia rolfsii/Zn2+ | In vivo experiments in mice showed that Ps–Zn complexes were more effective than inorganic and organic Zn supplements in treating Zn deficiency and improving antioxidant activities. | [86] | |
| Dictyophora indusiata; Prunella vulgaris/Zinc Chloride (ZnCl2); Zinc acetate (Zn(CH3CO2)2) | Ps–Zn complexes were reported to have significant anti-proliferative activity against a group of human cancer cell lines via several biological pathways. | [87,88] | |
| Fresh garlic/ZnSO4 | Ps–Zn complex could be considered as a potential form of Zn supplementation to alleviate the toxic effect induced by Zn deficiency in mice, including oxidative stress. | [89] | |
| Peptides (Pp) | Oyster/ZnSO4 | Pp-Zn complex could significantly enhance Zn bioavailability in vitro on Caco-2 cells and enhance Zn solubility during simulated gastrointestinal digestion in comparison to the commonly used ZnSO4. | [64,84] |
| Sea cucumber (stichopus ja ponicus); Scallop adductor (Patinopecten yessoensis)/ZnSO4 | In vitro experiments on Caco-2 cells suggest that marine-animal-derived peptides could be considered as a potential and safe Zn-chelating agents to enhance Zn absorption and bioavailability. | [90,91] | |
| Wheat/ZnSO4 | In vitro experiments on Caco-2 cells suggest that Zn-chelating peptides from wheat germ protein hydrolysates possessed higher Zn bioavailability than ZnSO4. | [92] |
6.2. Micro- and Nanoencapsulation of Zinc
- Zinc microencapsulation
- Zinc nanoencapsulation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Matteo, G.; Spano, M.; Grosso, M.; Salvo, A.; Ingallina, C.; Russo, M.; Ritieni, A.; Mannina, L. Food and COVID-19: Preventive/Co-Therapeutic Strategies Explored by Current Clinical Trials and in Silico Studies. Foods 2020, 9, 1036. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The Role of Zinc, Copper, Manganese and Iron in Neurodegenerative Diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Brazier, A.K.M.; Lowe, N.M. Zinc Deficiency in Low- and Middle-income Countries: Prevalence and Approaches for Mitigation. J. Hum. Nutr. Diet. 2020, 33, 624–643. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef] [Green Version]
- Maywald, M.; Rink, L. Zinc in Human Health and Infectious Diseases. Biomolecules 2022, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001; ISBN 0-309-07279-4.
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef] [Green Version]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the Zinc-Proteins Encoded in the Human Genome. J. Proteome Res. 2005, 5, 196–201. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, I.; Banni, M.; Saïd, L.; Saïd, K.; Kerkeni, A. Evaluation of Involvement of Testicular Metallothionein Gene Expression in the Protective Effect of Zinc against Cadmium-Induced Testicular Pathophysiology in Rat. Reprod. Toxicol. 2010, 29, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Chemek, M.; Minucci, S.; Messaoudi, I. Cadmium-induced Toxicity Increases Prolyl Endopeptidase (PREP) Expression in the Rat Testis. Mol. Reprod. Dev. 2020, 87, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Chemek, M.; Mimouna, S.B.; Boughammoura, S.; Delbès, G.; Messaoudi, I. Protective Role of Zinc against the Toxicity Induced by Exposure to Cadmium during Gestation and Lactation on Testis Development. Reprod. Toxicol. 2016, 63, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chemek, M.; Venditti, M.; Boughamoura, S.; Mimouna, S.B.; Messaoudi, I.; Minucci, S. Involvement of Testicular DAAM1 Expression in Zinc Protection against Cadmium-Induced Male Rat Reproductive Toxicity. J. Cell. Physiol. 2018, 233, 630–640. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and Its Importance for Human Health: An Integrative Review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [Green Version]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc Homeostasis in Humans. J. Nutr. 2000, 130, S1360–S1366. [Google Scholar] [CrossRef] [Green Version]
- Chemek, M.; Boughammoura, S.; Mimouna, S.B.; Chouchene, L.; Banni, M.; Messaoudi, I. Changes of the MRNA Expression Pattern of Zn Transporters: A Probable Mechanism for Cadmium Retention and Zinc Redistribution in the Suckling Rat Tissues. Biol. Trace Elem. Res. 2015, 165, 173–182. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, H.S.; El-Sayed, S.M.; Youssef, A.M. Novel Approach for Biosynthesizing of Zinc Oxide Nanoparticles Using Lactobacillus Gasseri and Their Influence on Microbiological, Chemical, Sensory Properties of Integrated Yogurt. Food Chem. 2021, 365, 130513. [Google Scholar] [CrossRef]
- Ma, X.; Qian, M.; Yang, Z.; Xu, T.; Han, X. Effects of Zinc Sources and Levels on Growth Performance, Zinc Status, Expressions of Zinc Transporters, and Zinc Bioavailability in Weaned Piglets. Animals 2021, 11, 2515. [Google Scholar] [CrossRef]
- Lim, K.H.C.; Riddell, L.J.; Nowson, C.A.; Booth, A.O.; Szymlek-Gay, E.A. Iron and Zinc Nutrition in the Economically-Developed World: A Review. Nutrients 2013, 5, 3184–3211. [Google Scholar] [CrossRef]
- Hall, A.G.; King, J.C. Zinc Fortification: Current Trends and Strategies. Nutrients 2022, 14, 3895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Revisiting Phytate-Element Interactions: Implications for Iron, Zinc and Calcium Bioavailability, with Emphasis on Legumes. Crit. Rev. Food Sci. Nutr. 2020, 62, 1696–1712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Anand, R. Effect of Germination and Temperature on Phytic Acid Content of Cereals. Int. J. Res. Agric. Sci. 2021, 8, 24–35. [Google Scholar]
- Hennigar, S.R.; McClung, J.P. Hepcidin Attenuates Zinc Efflux in Caco-2 Cells. J. Nutr. 2016, 146, 2167–2173. [Google Scholar] [CrossRef] [Green Version]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The Role of Fe, Zn, and Cu in Pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef]
- Jouanne, M.; Oddoux, S.; Noël, A.; Voisin-Chiret, A.S. Nutrient Requirements during Pregnancy and Lactation. Nutrients 2021, 13, 692. [Google Scholar] [CrossRef] [PubMed]
- Chemek, M.; Nevoral, J. The Dark Side of the Breastfeeding: In the Light of Endocrine Disruptors. Med. J. Cell Biol. 2019, 7, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Fenclová, T.; Chemek, M.; Havránková, J.; Kolinko, Y.; Sudová, V.; Moravec, J.; Navrátilová, J.; Klein, P.; Králíčková, M.; Nevoral, J. Effect of Bisphenol S on Testicular Tissue after Low-Dose Lactation Exposure. Environ. Pollut. 2022, 315, 120114. [Google Scholar] [CrossRef]
- Wessels, I.; Fischer, H.J.; Rink, L. Dietary and Physiological Effects of Zinc on the Immune System. Annu. Rev. Nutr. 2021, 41, 133–175. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc in Human Health: Effect of Zinc on Immune Cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S.; Malysa, A.; Bepler, G.; Fribley, A.; Bao, B. The Mechanisms of Zinc Action as a Potent Anti-Viral Agent: The Clinical Therapeutic Implication in COVID-19. Antioxidants 2022, 11, 1862. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Balsiger, L.M.; Maurizi, V.; Rinninella, E.; Gasbarrini, A.; Giostra, N.; Santori, P.; Abenavoli, L.; Rasetti, C. Zinc and Gut Microbiota in Health and Gastrointestinal Disease under the COVID-19 Suggestion. Biofactors 2022, 48, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, X. Epigenetic Regulation of the Innate Immune Response to Infection. Nat. Rev. Immunol. 2019, 19, 417–432. [Google Scholar] [CrossRef]
- Sen, R.; Barnes, C. Do Transgenerational Epigenetic Inheritance and Immune System Development Share Common Epigenetic Processes? J. Dev. Biol. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.P.; Abubakar, M.B.; Malami, I.; Ibrahim, K.G.; Abubakar, B.; Bello, M.B.; Qusty, N.; Elazab, S.T.; Imam, M.U.; Alexiou, A.; et al. Zinc Metalloproteins in Epigenetics and Their Crosstalk. Life 2021, 11, 186. [Google Scholar] [CrossRef]
- Kessels, J.E.; Wessels, I.; Haase, H.; Rink, L.; Uciechowski, P. Influence of DNA-Methylation on Zinc Homeostasis in Myeloid Cells: Regulation of Zinc Transporters and Zinc Binding Proteins. J. Trace Elem. Med. Biol. 2016, 37, 125–133. [Google Scholar] [CrossRef]
- Sadeghsoltani, F.; Mohammadzadeh, I.; Safari, M.-M.; Hassanpour, P.; Izadpanah, M.; Qujeq, D.; Moein, S.; Vaghari-Tabari, M. Zinc and Respiratory Viral Infections: Important Trace Element in Anti-Viral Response and Immune Regulation. Biol. Trace Elem. Res. 2022, 200, 2556–2571. [Google Scholar] [CrossRef]
- Galmés, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Brasiel, P.G. The Key Role of Zinc in Elderly Immunity: A Possible Approach in the COVID-19 Crisis. Clin. Nutr. ESPEN 2020, 38, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Derwand, R.; Scholz, M. Does Zinc Supplementation Enhance the Clinical Efficacy of Chloroquine/Hydroxychloroquine to Win Today’s Battle against COVID-19? Med. Hypotheses 2020, 142, 109815. [Google Scholar] [CrossRef]
- Chinni, V.; El-Khoury, J.; Perera, M.; Bellomo, R.; Jones, D.; Bolton, D.; Ischia, J.; Patel, O. Zinc Supplementation as an Adjunct Therapy for COVID-19: Challenges and Opportunities. Br. J. Clin. Pharmacol. 2021, 87, 3737–3746. [Google Scholar] [CrossRef]
- Finzi, E. Treatment of SARS-CoV-2 with High Dose Oral Zinc Salts: A Report on Four Patients. Int. J. Infect. Dis. 2020, 99, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Abdel-Bar, H.M.; Elmowafy, E.; El-Khouly, A.; Mansour, M.; Awad, G.A.S. Investigating the Internalization and COVID-19 Antiviral Computational Analysis of Optimized Nanoscale Zinc Oxide. ACS Omega 2021, 6, 6848–6860. [Google Scholar] [CrossRef]
- Hendi, A.A.; Virk, P.; Awad, M.A.; Elobeid, M.; Ortashi, K.M.O.; Alanazi, M.M.; Alkallas, F.H.; Almoneef, M.M.; Abdou, M.A. In Silico Studies on Zinc Oxide Based Nanostructured Oil Carriers with Seed Extracts of Nigella Sativa and Pimpinella Anisum as Potential Inhibitors of 3CL Protease of SARS-CoV-2. Molecules 2022, 27, 4301. [Google Scholar] [CrossRef]
- Clayton, H.S.; Ngoepe, M.P.; Tapala, K.C. Prophylactic and Therapeutic Potential Zinc Metallodrugs Drug Discovery: Identification of SARS-CoV-2 Replication and Spike/ACE2 Inhibitors. Curr. Comput. Aided Drug Des. 2022, 18, 519–534. [Google Scholar] [CrossRef]
- Lee, C.-C.; Kuo, C.-J.; Hsu, M.-F.; Liang, P.-H.; Fang, J.-M.; Shie, J.-J.; Wang, A.H.-J. Structural Basis of Mercury- and Zinc-Conjugated Complexes as SARS-CoV 3C-like Protease Inhibitors. FEBS Lett. 2007, 581, 5454–5458. [Google Scholar] [CrossRef] [Green Version]
- te Velthuis, A.J.W.; van den Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn(2+) Inhibits Coronavirus and Arterivirus RNA Polymerase Activity in Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Abomughaid, M.M.; Nofal, M.S.; Ghaleb, K.I.; Seadawy, M.G.; AbdEl-Wahab, M.G.; Hegazy, A.S.; Ghareeb, D.A. ZnO-Chlorogenic Acid Nanostructured Complex Inhibits COVID-19 Pathogenesis and Increases Hydroxychloroquine Efficacy. J. King Saud Univ. Sci. 2022, 34, 102296. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J. SARS-CoV-2 3CL-Protease Inhibitors as Antiviral Agent against COVID-19. Int. J. Appl. Pharm. 2022, 14, 18–20. [Google Scholar] [CrossRef]
- Alrabayah, I.N.; Elhawary, S.S.; Kandil, Z.A.; El-Kadder, E.M.A.; Moemen, Y.S.; Saleh, A.M.; El Raey, M.A. Green Synthesized Zinc Oxide Nanoparticles Based on Cestrum Diurnum L. of Potential Antiviral Activity against Human Corona 229-E Virus. Molecules 2022, 28, 266. [Google Scholar] [CrossRef]
- Ghareeb, D.A.; Saleh, S.R.; Seadawy, M.G.; Nofal, M.S.; Abdulmalek, S.A.; Hassan, S.F.; Khedr, S.M.; AbdElwahab, M.G.; Sobhy, A.A.; Abdel-Hamid, A.S.A.; et al. Nanoparticles of ZnO/Berberine Complex Contract COVID-19 and Respiratory Co-Bacterial Infection in Addition to Elimination of Hydroxychloroquine Toxicity. J. Pharm. Investig. 2021, 51, 735–757. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Emran, T.B.; Priyanaka; Choudhary, O.P. Immunomodulatory Effects of Zinc and Its Impact on COVID-19 Severity. Ann. Med. Surg. 2022, 77, 103638. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. COVID-19 Pandemic: Can Zinc Supplementation Provide an Additional Shield against the Infection? Comput. Struct. Biotechnol. J. 2021, 19, 1371–1378. [Google Scholar] [CrossRef]
- Schlesinger, L.; Arevalo, M.; Arredondo, S.; Diaz, M.; Lönnerdal, B.; Stekel, A. Effect of a Zinc-Fortified Formula on Immunocompetence and Growth of Malnourished Infants. Am. J. Clin. Nutr. 1992, 56, 491–498. [Google Scholar] [CrossRef]
- Costarelli, L.; Giacconi, R.; Malavolta, M.; Basso, A.; Piacenza, F.; DeMartiis, M.; Giannandrea, E.; Renieri, C.; Busco, F.; Galeazzi, R.; et al. Effects of Zinc-Fortified Drinking Skim Milk (as Functional Food) on Cytokine Release and Thymic Hormone Activity in Very Old Persons: A Pilot Study. Age 2014, 36, 9656. [Google Scholar] [CrossRef] [Green Version]
- Kanwar, A.; Sharma, A. A Review on Role of Zinc as a Potent Immunity Boosting Agent. Mater. Today Proc. 2022, 68, 880–885. [Google Scholar] [CrossRef]
- Nemzer, B.; Al-Taher, F.; Abshiru, N. Phytochemical Composition and Nutritional Value of Different Plant Parts in Two Cultivated and Wild Purslane (Portulaca Oleracea L.) Genotypes. Food Chem. 2020, 320, 126621. [Google Scholar] [CrossRef]
- Scherz, H.; Kirchhoff, E. Trace Elements in Foods: Zinc Contents of Raw Foods—A Comparison of Data Originating from Different Geographical Regions of the World. J. Food Compos. Anal. 2006, 19, 420–433. [Google Scholar] [CrossRef]
- Bilandžić, N.; Sedak, M.; Đokić, M.; Varenina, I.; Solomun Kolanović, B.; Božić, Đ.; Brstilo, M.; Šimić, B. Determination of Zinc Concentrations in Foods of Animal Origin, Fish and Shellfish from Croatia and Assessment of Their Contribution to Dietary Intake. J. Food Compos. Anal. 2014, 35, 61–66. [Google Scholar] [CrossRef]
- Li, J.; Gong, C.; Wang, Z.; Gao, R.; Ren, J.; Zhou, X.; Wang, H.; Xu, H.; Xiao, F.; Cao, Y.; et al. Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc. Mar. Drugs 2019, 17, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Imperio, M.; Durante, M.; Gonnella, M.; Renna, M.; Montesano, F.F.; Parente, A.; Mita, G.; Serio, F. Enhancing the Nutritional Value of Portulaca Oleracea L. by Using Soilless Agronomic Biofortification with Zinc. Food Res. Int. 2022, 155, 111057. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.J.; Wahbi, A.; Adu-Gyamfi, J.; Heiling, M.; Gruber, R.; Joy, E.J.M.; Broadley, M.R. Approaches to Reduce Zinc and Iron Deficits in Food Systems. Glob. Food Sec. 2017, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in Soils, Water and Food Crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Kamaral, C.; Neate, S.M.; Gunasinghe, N.; Milham, P.J.; Paterson, D.J.; Kopittke, P.M.; Seneweera, S. Genetic Biofortification of Wheat with Zinc: Opportunities to Fine-tune Zinc Uptake, Transport and Grain Loading. Physiol. Plant. 2021, 174, e13612. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Shkembi, B.; Huppertz, T. Influence of Dairy Products on Bioavailability of Zinc from Other Food Products: A Review of Complementarity at a Meal Level. Nutrients 2021, 13, 4253. [Google Scholar] [CrossRef]
- Lönnerdal, B. Dietary Factors Influencing Zinc Absorption. J. Nutr. 2000, 130, S1378–S1383. [Google Scholar] [CrossRef] [Green Version]
- Rosado, J.L. Zinc and Copper: Proposed Fortification Levels and Recommended Zinc Compounds. J. Nutr. 2003, 133, S2985–S2989. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lönnerdal, B.; Ruel, M.T.; Sandtröm, B.; Wasantwisut, E.; Hotz, C. International Zinc Nutrition Consultative Group (IZiNCG) Technical Document #1. Assessment of the Risk of Zinc Deficiency in Populations and Options for Its Control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar] [PubMed]
- Brown, K.H.; Hambidge, K.M.; Ranum, P. Zinc Fortification of Cereal Flours: Current Recommendations and Research Needs. Food Nutr. Bull. 2010, 31, S62–S74. [Google Scholar] [CrossRef] [PubMed]
- Wegmüller, R.; Tay, F.; Zeder, C.; Brnic, M.; Hurrell, R.F. Zinc Absorption by Young Adults from Supplemental Zinc Citrate Is Comparable with That from Zinc Gluconate and Higher than from Zinc Oxide. J. Nutr. 2014, 144, 132–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Du, L.; Liu, M.; Zhou, J.; Pan, W.; Fu, H.; Zhang, X.; Ma, Q.; Wu, L. Glycine-Chelated Zinc Rather than Glycine-Mixed Zinc Has Lower Foliar Phytotoxicity than Zinc Sulfate and Enhances Zinc Biofortification in Waxy Corn. Food Chem. 2022, 370, 131031. [Google Scholar] [CrossRef]
- Bashir, S.; Basit, A.; Abbas, R.N.; Naeem, S.; Bashir, S.; Ahmed, N.; Ahmed, M.S.; Ilyas, M.Z.; Aslam, Z.; Alotaibi, S.S.; et al. Combined Application of Zinc-Lysine Chelate and Zinc-Solubilizing Bacteria Improves Yield and Grain Biofortification of Maize (Zea Mays L.). PLoS ONE 2021, 16, e0254647. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; da Oliveira, C.E.S.; Fernandes, H.B.; Galindo, F.S.; da Silva, E.C.; Fernandes, G.C.; Nogueira, T.A.R.; De Carvalho, P.H.G.; Balbino, V.R.; de Lima, B.H.; et al. Diazotrophic Bacteria Is an Alternative Strategy for Increasing Grain Biofortification, Yield and Zinc Use Efficiency of Maize. Plants 2022, 11, 1125. [Google Scholar] [CrossRef]
- Miquel, E.; Farré, R. Effects and Future Trends of Casein Phosphopeptides on Zinc Bioavailability. Trends Food Sci. Technol. 2007, 18, 139–143. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of Enhancing Dietary Zinc Bioavailability with Food-Derived Zinc-Chelating Peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Tong, P.S.; Mao, X.Y. Zinc-Binding Capacity of Yak Casein Hydrolysate and the Zinc-Releasing Characteristics of Casein Hydrolysate-Zinc Complexes. J. Dairy Sci. 2011, 94, 2731–2740. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.; Sandström, B.; Lönnerdal, B. The Effect of Casein Phosphopetides on Zinc and Calcium Absorption from High Phytate Infant Diets Assessed in Rat Pups and Caco-2 Cells. Pediatr. Res. 1996, 40, 547–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulagesan, S.; Krishnan, S.; Nam, T.-J.; Choi, Y.-H. A Review of Bioactive Compounds in Oyster Shell and Tissues. Front. Bioeng. Biotechnol. 2022, 10, 913839. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Liu, X.; Zhao, M. Particulate Nanocomposite from Oyster (Crassostrea Rivularis) Hydrolysates via Zinc Chelation Improves Zinc Solubility and Peptide Activity. Food Chem. 2018, 258, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, B.; Ma, Y.; Chang, H.; Zheng, Z.; Zhao, X. Pumpkin Skin Polysaccharide-Zn(II) Complex: Preparation, Characterization, and Suppression of Inflammation in Zebrafish. Foods 2022, 11, 2610. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, H.; Min, W. Preparation, Characterization and Bioactivities of Athelia Rolfsii Exopolysaccharide-Zinc Complex (AEPS-Zinc). Int. J. Biol. Macromol. 2018, 113, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lu, Y.; Fu, J.; Ning, Z.; Yang, J.; Ren, J. Preparation and Characterization of Dictyophora Indusiata Polysaccharide–Zinc Complex and Its Augmented Antiproliferative Activity on Human Cancer Cells. J. Agric. Food Chem. 2015, 63, 6525–6534. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Xiao, J.; Fu, X.; You, L.; Liu, R.H. Preparation of Prunella Vulgaris Polysaccharide-Zinc Complex and Its Antiproliferative Activity in HepG2 Cells. Int. J. Biol. Macromol. 2016, 91, 671–679. [Google Scholar] [CrossRef]
- Bai, X.; Qiu, Z.; Zheng, Z.; Song, S.; Zhao, R.; Lu, X.; Qiao, X. Preparation and Characterization of Garlic Polysaccharide-Zn (II) Complexes and Their Bioactivities as a Zinc Supplement in Zn-Deficient Mice. Food Chem. X 2022, 15, 100361. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Ma, X.; Liu, X.; Yin, F.; Li, D.; Nakamura, Y.; Yu, C.; Zhou, D. Characterization of a Synthetic Zinc-chelating Peptide from Sea Cucumber (Stichopus Japonicus) and Its Gastrointestinal Digestion and Absorption in Vitro. J. Sci. Food Agric. 2022, 102, 4542–4550. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Wang, Z.; Yin, F.; Liu, H.; Nakamura, Y.; Yu, C.; Zhou, D. Gastrointestinal Digestion and Absorption Characterization in Vitro of Zinc-chelating Hydrolysate from Scallop Adductor (Patinopecten Yessoensis). J. Sci. Food Agric. 2021, 102, 3277–3286. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Wang, X.-P.; Guo, X.-N. Isolation and Characterization of Zinc-Chelating Peptides from Wheat Germ Protein Hydrolysates. J. Funct. Foods 2015, 12, 23–32. [Google Scholar] [CrossRef]
- Adinepour, F.; Pouramin, S.; Rashidinejad, A.; Jafari, S.M. Fortification/Enrichment of Milk and Dairy Products by Encapsulated Bioactive Ingredients. Food Res. Int. 2022, 157, 111212. [Google Scholar] [CrossRef] [PubMed]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Encapsulation of Carotenoid-Rich Oil from Gac Peel: Optimisation of the Encapsulating Process Using a Spray Drier and the Storage Stability of Encapsulated Powder. Powder Technol. 2019, 344, 373–379. [Google Scholar] [CrossRef]
- Baldelli, A.; Wells, S.; Pratap-Singh, A. Impact of Product Formulation on Spray-Dried Microencapsulated Zinc for Food Fortification. Food Bioprocess Technol. 2021, 14, 2286–2301. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Leiva, A. Double Fortified (Iron and Zinc) Spray-Dried Microencapsulated Premix for Food Fortification. LWT 2021, 151, 112189. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Zinc Oxide Nanoparticles for Food Packaging Applications. Antimicrob. Food Packag. 2016, 425–431. [Google Scholar] [CrossRef]
- Guan, Y.F.; Pedraza, A.J. Synthesis and Alignment of Zn and ZnO Nanoparticles by Laser-Assisted Chemical Vapor Deposition. Nanotechnology 2008, 19, 45609. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Al Jabri, H.; Saleem, M.H.; Rizwan, M.; Hussain, I.; Usman, K.; Alsafran, M. Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life 2022, 12, 594. [Google Scholar] [CrossRef]
- Alhujaily, M.; Albukhaty, S.; Yusuf, M.; Mohammed, M.K.A.; Sulaiman, G.M.; Al-Karagoly, H.; Alyamani, A.A.; Albaqami, J.; AlMalki, F.A. Recent Advances in Plant-Mediated Zinc Oxide Nanoparticles with Their Significant Biomedical Properties. Bioengineering 2022, 9, 541. [Google Scholar] [CrossRef]
- Ashwini, J.; Aswathy, T.R.; Achuthsankar, S.N. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Cayratia Pedata Leaf Extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef]
- Pouresmaeil, V.; Haghighi, S.; Raeisalsadati, A.S.; Neamati, A.; Homayouni-Tabrizi, M. The Anti-Breast Cancer Effects of Green-Synthesized Zinc Oxide Nanoparticles Using Carob Extracts. Anticancer Agents Med. Chem. 2021, 21, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Konkol, D.; Wojnarowski, K. The Use of Nanominerals in Animal Nutrition as a Way to Improve the Composition and Quality of Animal Products. J. Chem. 2018, 2018, 5927058. [Google Scholar] [CrossRef]
- Reshma, Z.; Meenal, K. Foliar Application of Biosynthesised Zinc Nanoparticles as a Strategy for Ferti-Fortification by Improving Yield, Zinc Content and Zinc Use Efficiency in Amaranth. Heliyon 2022, 8, e10912. [Google Scholar] [CrossRef]
- Elshayb, O.M.; Farroh, K.Y.; Amin, H.E.; Atta, A.M. Green Synthesis of Zinc Oxide Nanoparticles: Fortification for Rice Grain Yield and Nutrients Uptake Enhancement. Molecules 2021, 26, 584. [Google Scholar] [CrossRef] [PubMed]
- Murgueitio-Herrera, E.; Falconí, C.E.; Cumbal, L.; Gómez, J.; Yanchatipán, K.; Tapia, A.; Martínez, K.; Sinde-Gonzalez, I.; Toulkeridis, T. Synthesis of Iron, Zinc, and Manganese Nanofertilizers, Using Andean Blueberry Extract, and Their Effect in the Growth of Cabbage and Lupin Plants. Nanomaterials 2022, 12, 1921. [Google Scholar] [CrossRef]
- Santillán-Urquiza, E.; Méndez-Rojas, M.Á.; Vélez-Ruiz, J.F. Fortification of Yogurt with Nano and Micro Sized Calcium, Iron and Zinc, Effect on the Physicochemical and Rheological Properties. LWT 2017, 80, 462–469. [Google Scholar] [CrossRef]
- Karmakar, P.; Ray, P.R.; Chatterjee, P.N.; Mahato, A.; Haldar, L. Potential of Zinc Oxide Nanoparticle for Dietary Fortification in Yoghurt: Physicochemical, Microbiological, Rheological and Textural Analysis. Asian J. Dairy Food Res. 2022, 39, 175–180. [Google Scholar] [CrossRef]
- Hosseini-Vardanjani, S.F.; Rezaei, J.; Karimi-Dehkordi, S.; Rouzbehan, Y. Effect of Feeding Nano-ZnO on Performance, Rumen Fermentation, Leukocytes, Antioxidant Capacity, Blood Serum Enzymes and Minerals of Ewes. Small Rumin. Res. 2020, 191, 106170. [Google Scholar] [CrossRef]
- Cai, J.; Miao, C.; Chen, Y.; Xie, Y.; Liu, J.; Wang, D. Nano-Sized Zinc Addition Enhanced Mammary Zinc Translocation without Altering Health Status of Dairy Cows. Anim. Nutr. 2021, 7, 1024–1030. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Diab, A.M.; ElSaied, B.E.F.; Tayel, A.A.; Moussa, S.H. Application of ZnO Nanoparticles Phycosynthesized with Ulva Fasciata Extract for Preserving Peeled Shrimp Quality. Nanomaterials 2021, 11, 385. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.A.; Amer, S.A.; Mobashar, M.; El-Sayed, H.G.M. Use of Zinc Oxide Nanoparticles in the Growing Rabbit Diets to Mitigate Hot Environmental Conditions for Sustainable Production and Improved Meat Quality. BMC Vet. Res. 2022, 18, 354. [Google Scholar] [CrossRef] [PubMed]
- Khajeh Bami, M.; Afsharmanesh, M.; Ebrahimnejad, H. Effect of Dietary Bacillus Coagulans and Different Forms of Zinc on Performance, Intestinal Microbiota, Carcass and Meat Quality of Broiler Chickens. Probiotics Antimicrob. Proteins 2019, 12, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Influence of Dietary Biosynthesized Zinc Oxide Nanoparticles on Broiler Zinc Uptake, Bone Quality, and Antioxidative Status. Animals 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Bribiesca, J.E.; Casas, R.L.; Cruz Monterrosa, R.G.; Pérez, A.R. Supplementing Selenium and Zinc Nanoparticles in Ruminants for Improving Their Bioavailability Meat. Nutr. Deliv. 2017, 713–747. [Google Scholar] [CrossRef]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano Zinc, an Alternative to Conventional Zinc as Animal Feed Supplement: A Review. Anim. Nutr. 2016, 2, 134–141. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Mao, S.Y.; Li, M.Z.; Huang, J.T.; Lien, T.F. Effects of Nanosize Zinc Oxide on Zinc Retention, Eggshell Quality, Immune Response and Serum Parameters of Aged Laying Hens. Anim. Feed Sci. Technol. 2016, 213, 99–107. [Google Scholar] [CrossRef]
- Youn, S.-M.; Choi, S.-J. Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity. Int. J. Mol. Sci. 2022, 23, 6074. [Google Scholar] [CrossRef]

| Food Product Fortified/Biofortified with Zn NPs | Fortification/Biofortification Methods | Consequence of Food Fortification/Biofortification with Zn NPs | References |
|---|---|---|---|
| Cereal-based products (rice, wheat, etc.) | Biofortification (Foliar fortification) | Foliar application of Zn NPs (synthetized by green technology) at low and moderate doses enhanced the growth, yield, and the quality of some cereal crops (rice, wheat, and amaranth). | [105,106,107] |
| Dairy-based products (milk, yoghurt, etc.) | Fortification | Fortification of dairy products with Zn NPs showed advantages over conventional processes in terms of microbial profile, physicochemical, and rheological properties right after manufacturing and during refrigerated storage. In vitro digestion analysis of the dairy product fortified with Zn NPs showed more solubility than conventional fortification. | [21,108,109] |
| Biofortification | The administration of Zn NPs in cattle feed reduces the number of somatic cells in milk, increases Zn concentration, and improves milk production in comparison with conventional Zn supplementation. | [104,110,111] | |
| Meat- and fish-based products | Fortification | The uses of Zn NPs (synthetized by green technology) enhance shrimps’ biopreservation during refrigerated storage by improving sensorial qualities and decreasing the microbial profile of the animal product. | [112] |
| Biofortification | Results indicated that feeding animals (rabbits, pigs, etc.) with Zn NPs increases Zn absorption and bioavailability in comparison to regular Zn sources and has a positive effect on production efficiency, quality, and characteristics (physicochemical properties, antioxidant status, Zn content, etc.) of eggs, meat, and bones of feeding animals. | [113,114,115,116,117,118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chemek, M.; Kadi, A.; Merenkova, S.; Potoroko, I.; Messaoudi, I. Improving Dietary Zinc Bioavailability Using New Food Fortification Approaches: A Promising Tool to Boost Immunity in the Light of COVID-19. Biology 2023, 12, 514. https://doi.org/10.3390/biology12040514
Chemek M, Kadi A, Merenkova S, Potoroko I, Messaoudi I. Improving Dietary Zinc Bioavailability Using New Food Fortification Approaches: A Promising Tool to Boost Immunity in the Light of COVID-19. Biology. 2023; 12(4):514. https://doi.org/10.3390/biology12040514
Chicago/Turabian StyleChemek, Marouane, Ammar Kadi, Svetlana Merenkova, Irina Potoroko, and Imed Messaoudi. 2023. "Improving Dietary Zinc Bioavailability Using New Food Fortification Approaches: A Promising Tool to Boost Immunity in the Light of COVID-19" Biology 12, no. 4: 514. https://doi.org/10.3390/biology12040514
APA StyleChemek, M., Kadi, A., Merenkova, S., Potoroko, I., & Messaoudi, I. (2023). Improving Dietary Zinc Bioavailability Using New Food Fortification Approaches: A Promising Tool to Boost Immunity in the Light of COVID-19. Biology, 12(4), 514. https://doi.org/10.3390/biology12040514