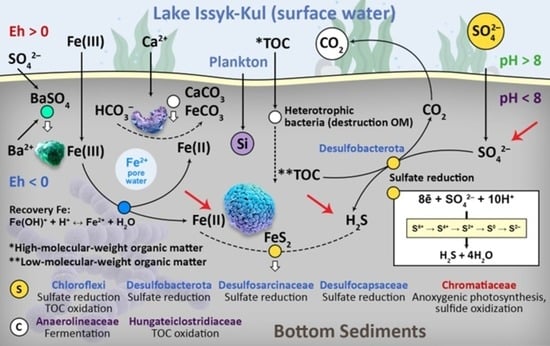
Microbial Diversity and Authigenic Mineral Formation of Modern Bottom Sediments in the Littoral Zone of Lake Issyk-Kul, Kyrgyz Republic (Central Asia)
Abstract
:Simple Summary
Abstract
1. Introduction
- To study the biogeochemistry of modern bottom sediments in the littoral zone of Lake Issyk-Kul;
- Determine the phylogenetic diversity of microbial communities of bottom sediments based on 16S rRNA gene sequence analysis;
- Identify authigenic minerals in bottom sediments and relate the diversity of different physiological groups of microorganisms in biogeochemical processes of the littoral zone (organic matter decomposition, chemical elements distribution, pore water transformation, etc.).
2. Physiographic Background and Methods
2.1. Physiographic Background
2.2. Research Methods
2.2.1. Sampling
2.2.2. Analytical Methods
2.2.3. High-Throughput Sequencing of 16S rRNA Genes
2.2.4. Geochemical Modelling
3. Results
3.1. Physico-Chemical Characteristics of Lake and Pore Waters
3.2. Geochemical Characteristics of Bottom Sediments
3.3. Authigenic Minerals
3.4. Microbial Diversity Analysis in Bottom Sediments
4. Discussion
5. Conclusions
- i.
- The activities of heterotrophic and sulphate-reducing microorganisms may produce authigenic carbonate minerals (calcite);
- ii.
- The activity of sulphate-reducing bacteria may provide the basis for the growth of reduced sulphur forms and the formation of authigenic sulphide minerals (rambogenic pyrite);
- iii.
- The activity of sulphate-reducing bacteria can lead to the deposition of barite at the redox boundary;
- iv.
- The activity of heterotrophic microorganisms can lead to a decrease in pH (due to the decomposition of organic matter), which creates conditions for the deposition of amorphous silica.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadd, G.M.; Raven, J.A. Geomicrobiology of eukaryotic microorganisms. Geomicrobiol. J. 2010, 27, 491–519. [Google Scholar] [CrossRef]
- Ivanov, M.V.; Karavaiko, G.I. Geological Microbiology. Microbiology 2004, 73, 493–508. [Google Scholar] [CrossRef]
- Renshaw, J. Introduction to Geomicrobiology—By K. Konhauser. Eur. J. Soil Sci. 2007, 58, 1215–1216. [Google Scholar] [CrossRef]
- Zavarzin, G.A. A planet of bacteria. Her. Russ. Acad. Sci. 2008, 78, 2. [Google Scholar] [CrossRef]
- Teske, A.; Biddle, J.F.; Edgcomb, V.P.; Schippers, A. Deep subsurface microbiology: A guide to the research topic papers. Front. Microbiol. 2013, 4, 122. [Google Scholar] [CrossRef] [Green Version]
- Bryanskaya, A.V.; Shipova, A.A.; Rozanov, A.S.; Kolpakova, O.A.; Lazareva, E.V.; Uvarova, Y.E.; Efimov, V.M.; Zhmodik, S.M.; Taran, O.P.; Goryachkovskaya, T.N.; et al. Diversity and Metabolism of Microbial Communities in a Hypersaline Lake along a Geochemical Gradient. Biology 2022, 11, 605. [Google Scholar] [CrossRef]
- Kompantseva, E.I.; Bryantseva, I.A.; Komova, A.V.; Namsaraev, B.B. The structure of phototrophic communities of soda lakes of the southeastern Transbaikal Region. Microbiology 2007, 76, 2. [Google Scholar] [CrossRef]
- Vavourakis, C.D.; Andrei, A.-S.; Mehrshad, M.; Ghai, R.; Sorokin, D.Y.; Muyzer, G. A metagenomics roadmap to the uncultured genome diversity in hypersaline soda lake sediments. Microbiome 2018, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Zakharyuk, A.G.; Kozyreva, L.P.; Namsaraev, B.B. Abundance and Activity of the Bacteria-Destructors of Organic Matter in Saline-and-Soda Lake Khilganta (South Transbaikalia) in the pH-Salinity Gradient. Contemp. Probl. Ecol. 2010, 3, 4. [Google Scholar] [CrossRef]
- Leonova, G.A.; Maltsev, A.E.; Melenevsky, V.N.; Krivonogov, S.K.; Kondratyeva, L.M.; Bobrov, V.A.; Suslova, M.Y. Diagenetic transformation of organic matter in sapropel sediments of small lakes (southern West Siberia and eastern Transbaikalia). Quat. Int. 2019, 524, 40–47. [Google Scholar] [CrossRef]
- Zavarzin, G.A. Microbial Geochemical Calcium Cycle. Microbiology 2002, 71, 1–17. [Google Scholar] [CrossRef]
- Maltsev, A.; Safonov, A.V.; Leonova, G.; Krivonogov, S. Role of microorganisms in destruction of organic matter and processes of diagenetic mineral formation in the sediments of Western Siberia lakes Ion selective electrodes View project Radioecology and biogeochemistry of subsurface waters. In IV All-Russian Scientific Conference with international participation; Springer: Berlin/Heidelberg, Germany, 2022; p. 356. [Google Scholar]
- Podrezov, A.O.; Mäkelä, A.J.; Mischke, S. Lake Issyk-Kul: Its History and Present State. In Large Asian Lakes in a Changing World; Springer: Berlin/Heidelberg, Germany, 2020; pp. 177–206. [Google Scholar]
- Braissant, O.; Verrecchia, E.P. Microbial biscuits of Vaterite in Lake Issyk-Kul (republic of Kyrgyzstan)-discussion. J. Sediment. Res. 2002, 72, 6. [Google Scholar] [CrossRef]
- Rojas-Jimenez, K.; Araya-Lobo, A.; Quesada-Perez, F.; Akerman-Sanchez, J.; Delgado-Duran, B.; Ganzert, L.; Zavialov, P.O.; Alymkulov, S.; Kirillin, G.; Grossart, H. Variation of bacterial communities along the vertical gradient in Lake Issyk Kul, Kyrgyzstan. Environ. Microbiol. Rep. 2021, 13, 337–347. [Google Scholar] [CrossRef]
- Kozhokulov, S.; Issanova, G.; Chen, X. Ecological Problems in the Issyk-Kul Region. In Tourism in the Kyrgyz Republic; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 123–131. [Google Scholar]
- Konurbaeva, M.U. Hydrocarbon-oxidizing ability of microorganisms isolated from oil-polluted oil products of balykchy coastal soil. In Waste, Causes of Their Formation and Prospects for Use; Kuban State Agrarian University: Krasnodar, Russia, 2019; pp. 343–344. [Google Scholar]
- Samylina, O.S.; Zaytseva, L.V. Characterization of modern dolomite stromatolites from hypersaline Petukhovskoe Soda Lake, Russia. Lethaia 2019, 52, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Samylina, O.S.; Namsaraev, Z.B.; Grouzdev, D.S.; Slobodova, N.V.; Zelenev, V.V.; Borisenko, G.V.; Sorokin, D.Y. The patterns of nitrogen fixation in haloalkaliphilic phototrophic communities of Kulunda Steppe soda lakes (Altai, Russia). FEMS Microbiol. Ecol. 2019, 95, 11. [Google Scholar] [CrossRef]
- Sapozhnikov, P.V.; Kalinina, O.Y.; Nikitin, M.A.; Samylina, O.S. Cenoses of phototrophic algae of ultrasaline lakes in the Kulunda steppe (Altai krai, Russian Federation). Oceanology 2016, 56, 95–106. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Merkel, A.Y.; Messina, E.; Tugui, C.; Pabst, M.; Golyshin, P.N.; Yakimov, M.M. Anaerobic carboxydotrophy in sulfur-respiring haloarchaea from hypersaline lakes. ISME J. 2022, 16, 1534–1546. [Google Scholar] [CrossRef]
- Nihoul, J.C.; Zavialov, P.O.; Micklin, P.P. Dying and Dead Seas Climatic versus Anthropic Causes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004; p. 36. [Google Scholar]
- Romanovsky, V.V. Water Level Variations and Water Balance of Lake Issyk-Kul. In Lake Issyk-Kul: Its Natural Environment; Springer: Dordrecht, The Netherlands, 2002; pp. 45–57. [Google Scholar]
- Batist, M.; Imbo, Y.; Vermeesch, P.; Klerkx, J.; Giralt, S.; Delvaux, D.; Lignier, V.; Beck, C.; Kalugin, I.; Abdrakhmatov, K.E. Bathymetry and Sedimentary Environments of Lake Issyk-Kul, Kyrgyz Republic (Central Asia): A Large, High-Altitude, Tectonic Lake. In Lake Issyk-Kul: Its Natural Environment; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Giralt, S.; Klerkx, J.; Riera, S.; Julia, R.; Lignier, V.; Beck, C.; Batist, M.; Kalugin, I. Recent Paleoenvironmental Evolution of Lake Issyk-Kul. In Lake Issyk-Kul: Its Natural Environment; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Titova, Y.A. Granulometric composition of bottom sediments of Issyk-Kul Lake. Bull. Kyrg. Natl. Agrar. Univ. Named K.I. Skryabin 2021, 1, 106–109. [Google Scholar]
- Giralt, S.; Julià, R.; Klerkx, J.; Riera, S.; Leroy, S.; Buchaca, T.; Catalan, J.; de Batist, M.; Beck, C.; Bobrov, V.; et al. 1000-Year Environmental History of Lake Issyk-Kul. In Dying and Dead Seas Climatic versus Anthropic Causes; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Fadeeva, V.P.; Tikhova, V.D.; Nikulicheva, O.N. Elemental analysis of organic compounds with the use of automated CHNS analyzers. J. Anal. Chem. 2008, 63, 11. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Fiori, C.; Lifshin, E. Materials Specimen Preparation for SEM and X-ray Microanalysis. In Scanning Electron Microscopy and X-ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D1. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 3. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s guide to Phreeqc (version 2)-a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In Water-Resources Investigations Report; U.S. Geological Survey: New York, NY, USA, 1999. [Google Scholar]
- Asankulov, T.; Abuduwaili, J.; Issanova, G.; Long, M.; Duulatov, E. Long-Term Dynamics and Seasonal Changes in Hydrochemistry of the Issyk-Kul Lake Basin, Kyrgyzstan. Arid Ecosyst. 2019, 9, 69–76. [Google Scholar] [CrossRef]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T.; Garrity, G.M.; Brenner, D.J.; de Vos, P.; Garrity, G.M.; Goodfellow, M.; Noel, R. Volume 2: The Proteobacteria, Part B: The Gammaproteobacteria. In Bergey’s Manual of Systematic Bacteriology; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Teske, A.; Biddle, J.F.; Edgcomb, V.P.; Schippers, A. Deep Subsurface Microbiology. Frontiers Media SA. Front. Res. Found. 2015. [Google Scholar] [CrossRef]
- Yamada, T.; Sekiguchi, Y. Anaerolineaceae. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2018; pp. 1–5. [Google Scholar]
- Watanabe, M.; Higashioka, Y.; Kojima, H.; Fukui, M. Desulfosarcina widdelii sp. nov. and Desulfosarcina alkanivorans sp. nov., hydrocarbon-degrading sulfate-reducing bacteria isolated from marine sediment and emended description of the genus Desulfosarcina. Int. J. Syst. Evol. Microbiol. 2017, 67, 8. [Google Scholar] [CrossRef] [PubMed]
- Galushko, A.; Kuever, J. Desulfocapsaceae. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 1–6. [Google Scholar]
- Dubinina, G.; Grabovich, M.; Leshcheva, N.; Rainey, F.A.; Gavrish, E. Spirochaeta perfilievii sp. nov., an oxygen-tolerant, sulfide-oxidizing, sulfur- and thiosulfate-reducing spirochaete isolated from a saline spring. Int. J. Syst. Evol. Microbiol. 2011, 61, 110–117. [Google Scholar] [CrossRef] [Green Version]
- West-Roberts, J.A.; Matheus-Carnevali, P.B.; Schoelmerich, M.C.; Al-Shayeb, B.; Thomas, A.D.; Sharrar, A.; He, C.; Chen, L.-X.; Lavy, A.; Keren, R.; et al. The Chloroflexi supergroup is metabolically diverse and representatives have novel genes for non-photosynthesis based CO2 fixation. BioRxiv 2021. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, D.; Zhou, H.; Gao, H.; Li, J. Sulfate reduction and formation of iron sulfide minerals in nearshore sediments from Qi’ao Island, Pearl River Estuary, Southern China. Quat. Int. 2017, 452, 137–147. [Google Scholar] [CrossRef]
- Yabe, S.; Sakai, Y.; Abe, K.; Yokota, A. Diversity of Ktedonobacteria with Actinomycetes-Like Morphology in Terrestrial Environments. Microbes Environ. 2017, 32, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, D.; Sauro, F.; Columbu, A.; Carbone, C.; Hong, P.-Y.; Vergara, F.; de Waele, J.; Cappelletti, M. Transition from unclassified Ktedonobacterales to Actinobacteria during amorphous silica precipitation in a quartzite cave environment. Sci. Rep. 2021, 11, 3921. [Google Scholar] [CrossRef] [PubMed]
- Kizina, J.; Jordan, S.F.A.; Martens, G.A.; Lonsing, A.; Probian, C.; Kolovou, A.; Santarella-Mellwig, R.; Rhiel, E.; Littmann, S.; Markert, S.; et al. Methanosaeta and “ Candidatus Velamenicoccus archaeovorus”. Appl. Environ. Microbiol. 2022, 88, 7. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Pronina, N.A. Carbonic anhydrase: Enzyme that has transformed the biosphere. Russ. J. Plant Physiol. 2011, 58, 197–209. [Google Scholar] [CrossRef]
- Zavarzin, G.A.; Zhilina, T.N. Anaerobic Chemotrophic Alkaliphiles. In Journey to Diverse Microbial Worlds; Springer: Dordrecht, The Netherlands, 2000; pp. 191–208. [Google Scholar]
- Pesciaroli, C.; Purswani, J.; Mestelan, S.; Lett, L.; Portela, G.; Medici, S.; Morillo, J.A.; Pozo, C.; González-López, J.; Rivadeneyra, M.A. Bacterial Diversity in Calcium Carbonate Paleo Accretions (Tosca) in the Southern Pampas, Argentina. Geomicrobiol. J. 2021, 38, 869–878. [Google Scholar] [CrossRef]
- Sergeev, V.N.; Gerasimenko, L.M.; Zavarzin, G.A. The Proterozoic History and Present State of Cyanobacteria. Microbiology 2002, 71, 623–637. [Google Scholar] [CrossRef]
- Zaitseva, L.V.; Orleanskii, V.K.; Gerasimenko, L.M.; Ushatinskaya, G.T. The role of cyanobacteria in crystallization of magnesium calcites. Paleontol. J. 2006, 40, 125–133. [Google Scholar] [CrossRef]
- Ward, D.M.; Ferris, M.J.; Nold, S.C.; Bateson, M.M. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol. Mol. Biol. Rev. 1998, 62, 1353–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandbeck, K.A.; Ward, D.M. Temperature Adaptations in the Terminal Processes of Anaerobic Decomposition of Yellowstone National Park and Icelandic Hot Spring Microbial Mats. Appl. Environ. Microbiol. 1982, 44, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Ward, L.G.; Michael Kemp, W.; Boynton, W.R. The influence of waves and seagrass communities on suspended particulates in an estuarine embayment. Mar. Geol. 1984, 59, 85–103. [Google Scholar] [CrossRef]
- Savvichev, A.S.; Lunina, O.N.; Rusanov, I.I.; Zakharova, E.E.; Veslopolova, E.F.; Ivanov, M.V. Microbiological and isotopic geochemical investigation of Lake Kislo-Sladkoe, a meromictic water body at the Kandalaksha Bay shore (White Sea). Microbiology 2014, 83, 56–66. [Google Scholar] [CrossRef]
- Kohn, M.J.; Riciputi, L.R.; Stakes, D.; Orange, D.L. Sulfur isotope variability in biogenic pyrite; reflections of heterogeneous bacterial colonization? Am. Mineralogist. 1998, 83, 1454–1468. [Google Scholar] [CrossRef]
- Farrand, M. Framboidal sulphides precipitated synthetically. Miner. Depos. 1970, 5, 237–247. [Google Scholar] [CrossRef]
- Folk, R.L. Nannobacteria and the formation of framboidal pyrite: Textural evidence. J. Earth Syst. Sci. 2005, 114, 369–374. [Google Scholar] [CrossRef]
- Gonneea, M.E.; Paytan, A. Phase associations of barium in marine sediments. Mar. Chem. 2006, 100, 124–135. [Google Scholar] [CrossRef]
- Griffith, E.M.; Paytan, A. Barite in the ocean—Occurrence, geochemistry and palaeoceanographic applications. Sedimentology 2012, 59, 1817–1835. [Google Scholar] [CrossRef]
- Riedinger, N.; Kasten, S.; Gröger, J.; Franke, C.; Pfeifer, K. Active and buried authigenic barite fronts in sediments from the Eastern Cape Basin. Earth Planet Sci. Lett. 2006, 241, 876–887. [Google Scholar] [CrossRef]
- Stamatakis, M.G.; Hein, J.R. Origin of barite in Tertiary marine sedimentary rocks from Lefkas Island, Greece. Econ. Geol. 1993, 88, 91–103. [Google Scholar] [CrossRef]
- Ganeshram, R.S.; François, R.; Commeau, J.; Brown-Leger, S.L. An experimental investigation of barite formation in seawater. Geochim. Cosmochim. Acta 2003, 67, 2599–2605. [Google Scholar] [CrossRef]
- Abakumov, A.I.; Pak, S.Y.; Morozov, M.A.; Tynybekov, A.K. Model Estimation of the Phytoplankton Biomass of Lake Issyk-Kul Using Remote Sensing Data. Inland Water Biol. 2019, 12, 112–118. [Google Scholar] [CrossRef]
- McManus, J.; Berelson, W.M.; Klinkhammer, G.P.; Johnson, K.S.; Coale, K.H.; Anderson, R.F.; Kumar, N.; Burdige, D.J.; Hammond, D.E.; Brumsack, H.J.; et al. Geochemistry of barium in marine sediments: Implications for its use as a paleoproxy. Geochim. Cosmochim. Acta 1998, 62, 3453–3473. [Google Scholar] [CrossRef]







| Sample/Depth | TC | TOC | H | N | S Total | S (VI) | S (II) |
|---|---|---|---|---|---|---|---|
| IK-1, 0–5 cm | 2.56 | 0.9 | <0.3 | <0.3 | 0.11 | 0.03 | 0.08 |
| IK-1, 25–30 cm | 1.66 | 0.2 | <0.3 | <0.3 | 0.15 | 0.01 | 0.14 |
| IK-2 | 2.66 | 0.5 | 0.40 | <0.3 | 0.19 | 0.02 | 0,17 |
| IK-3 | 4.25 | 1.9 | 0.52 | 0.45 | 0.18 | 0.02 | 0.16 |
| IK-4 | 1.13 | 0.5 | <0.3 | <0.3 | 0.26 | 0.08 | 0.18 |
| IK-5 | 4.15 | 1.1 | 0.48 | <0.3 | 0.32 | 0.03 | 0.29 |
| Sample/Depth | Al2O3 | CaO | Fe2O3 | K2O | MgO | MnO | Na2O | SiO2 | Ba | Mo | Sr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | ppm | ppm | ppm | |
| IK-1, 0–5 cm | 12.7 | 10.4 | 3.31 | 2.82 | 2.21 | 0.063 | 3.05 | 52.2 | 651 | 11.6 | 813.1 |
| IK-1, 25–30 cm | 13.8 | 7.0 | 3.64 | 2.99 | 2.22 | 0.066 | 3.34 | 65.8 | 687 | 5.5 | 333.1 |
| IK-2 | 15.1 | 9.0 | 4.13 | 3.46 | 2.12 | 0.076 | 2.80 | 57.9 | 658 | 23.1 | 467.9 |
| IK-3 | 12.0 | 8.3 | 3.96 | 2.61 | 2.55 | 0.072 | 2.20 | 56.8 | 513 | 4.0 | 542.3 |
| IK-4 | 12.8 | 7.5 | 5.22 | 3.04 | 1.93 | 0.097 | 2.88 | 54.6 | 801 | 47.0 | 511.2 |
| IK-5 | 11.8 | 14.3 | 3.87 | 2.71 | 3.48 | 0.072 | 1.90 | 45.5 | 581 | 19.8 | 599.8 |
| Mineral Phase | IK-1 | IK-2 | IK-3 | IK-4 | IK-5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| W | BS | W | BS | W | BS | W | BS | W | BS | |
| Anhydrite CaSO4 | −4.71 | −4.71 | −5.06 | −13.86 | −6.53 | −6.53 | −5.00 | −10.28 | −4.66 | −4.66 |
| Barite BaSO4 * | 1.73 | 1.72 | 1.81 | −6.98 | −0.13 | −0.13 | 1.75 | −3.55 | 1.59 | 1.59 |
| Aragonite CaCO3 | −2.34 | −2.34 | −3.05 | −3.05 | −3.93 | −3.93 | −2.96 | −2.95 | −2.48 | −2.48 |
| Calcite CaCO3 * | −2.19 | −2.19 | −2.91 | −2.90 | −3.78 | −3.78 | −2.81 | −2.81 | −2.33 | −2.33 |
| Cerussite PbCO3 | 0.18 | 0.19 | −0.83 | −0.82 | −1.84 | −1.83 | −0.37 | −0.34 | −0.50 | −0.48 |
| Magnesite MgCO3 | 0.25 | −0.02 | −0.67 | −0.67 | −1.47 | −1.46 | −0.34 | −0.33 | −0.26 | −0.26 |
| Witherite BaCO3 | 3.42 | 3.41 | 3.14 | 3.15 | 1.79 | 1.79 | 3.10 | 3.10 | 3.09 | 3.09 |
| Strontianite SrCO3 | 0.97 | 0.97 | 0.31 | 0.31 | −0.76 | −0.76 | 0.65 | 0.65 | 0.55 | 0.55 |
| Strengite FePO4⋅2H2O | 0.67 | −6.54 | −0.29 | −7.85 | 2.11 | −4.29 | −0.32 | −7.22 | 0.19 | −5.55 |
| Fe(OH)3 | 2.49 | −3.37 | 2.49 | −5.07 | 2.59 | −3.81 | 2.71 | −4.17 | 2.59 | −3.15 |
| Goethite FeOOH | 7.63 | 1.77 | 7.63 | 0.07 | 7.73 | 1.33 | 7.84 | 0.96 | 7.73 | 1.99 |
| Hematite Fe2O3 | 16.24 | 0.98 | 16.24 | −0.01 | 16.43 | 0.23 | 16.66 | −0.12 | 16.44 | 0.23 |
| Fe(OH)2 | −5.18 | −1.92 | −5.17 | −2.30 | −5.07 | −3.86 | −5.11 | −1.83 | −4.50 | −2.37 |
| Magnetite Fe3O4 | 14.61 | 6.16 | 14.63 | 2.38 | 14.86 | 3.33 | 15.11 | 4.63 | 15.49 | 6.15 |
| Pyrite FeS2 * | −127.21 | 3.87 | −126.86 | 4.44 | −125.17 | −17.82 | −129.22 | 5.74 | −117.31 | −5.05 |
| Pyrrhotite FeS | −76.11 | 0.20 | −75.90 | 1.61 | −74.99 | −13.13 | −77.22 | 2.07 | −70.23 | −5.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivonogov, S.; Maltsev, A.; Zelenina, D.; Safonov, A. Microbial Diversity and Authigenic Mineral Formation of Modern Bottom Sediments in the Littoral Zone of Lake Issyk-Kul, Kyrgyz Republic (Central Asia). Biology 2023, 12, 642. https://doi.org/10.3390/biology12050642
Krivonogov S, Maltsev A, Zelenina D, Safonov A. Microbial Diversity and Authigenic Mineral Formation of Modern Bottom Sediments in the Littoral Zone of Lake Issyk-Kul, Kyrgyz Republic (Central Asia). Biology. 2023; 12(5):642. https://doi.org/10.3390/biology12050642
Chicago/Turabian StyleKrivonogov, Sergei, Anton Maltsev, Darya Zelenina, and Alexey Safonov. 2023. "Microbial Diversity and Authigenic Mineral Formation of Modern Bottom Sediments in the Littoral Zone of Lake Issyk-Kul, Kyrgyz Republic (Central Asia)" Biology 12, no. 5: 642. https://doi.org/10.3390/biology12050642
APA StyleKrivonogov, S., Maltsev, A., Zelenina, D., & Safonov, A. (2023). Microbial Diversity and Authigenic Mineral Formation of Modern Bottom Sediments in the Littoral Zone of Lake Issyk-Kul, Kyrgyz Republic (Central Asia). Biology, 12(5), 642. https://doi.org/10.3390/biology12050642