Drought, Deluge and Declines: The Impact of Precipitation Extremes on Amphibians in a Changing Climate
Abstract
:1. Introduction
2. Overview of Climate Change Effects on Amphibians and Other Herpetofauna
2.1. Effects of Drought on Amphibians
2.2. Effects of Deluge from Major Storm Events on Amphibians
2.3. Rainfall Pulses versus Total Amount of Precipitation
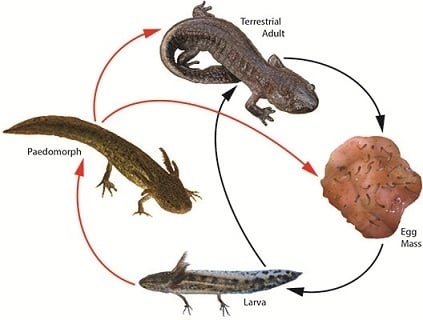
3. Landscape and Metapopulation-level Effects of Extreme Climatic Events: An Example with the Mole Salamander, Ambystoma talpoideum


4. Conclusions
Acknowledgments
References
- Travis, J.M.J. Climate change and habitat destruction: a deadly anthropogenic cocktail. Proc. R. Soc. B 2003, 270, 467–473. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Bickford, D.; Diesmos, A.C.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Sekercioglu, C.H.; Bradshaw, C.J.A. Measuring the meltdown: drivers of global amphibian extinction and decline. PloS Biol. 2008, 3, e1636. [Google Scholar]
- Lawler, J.J.; Shafer, S.L.; White, D.; Kareiva, P.; Maurer, E.P.; Blaustein, A.R.; Baratlein, P.J. Projected climate-induced faunal change in the Western Hemisphere. Ecology 2009, 90, 588–597. [Google Scholar] [CrossRef]
- Mainka, S.A.; Howard, G.W. Climate change and invasive species: double jeopardy. Integr. Zool. 2010, 5, 102–111. [Google Scholar] [CrossRef]
- Hof, C.; Araújo, M.B.; Jetz, W.; Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 2011, 480, 516–519. [Google Scholar]
- Bishop, P.J.; Angulo, A.; Lewis, J.P.; Moore, R.D.; Rabb, G.B.; Moreno, J.G. The amphibian extinction crisis—What will it take to put the action into the amphibian conservation action plan? SAPIENS 2012, 5, 97–111. [Google Scholar]
- Mantyka-Pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Glob. Change Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.J.; Searle, C.L.; Gervasi, S.S. Direct and indirect effects of climate change on amphibian populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. P. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; Hughes, L.; Huntley, B.; van Jaarsveld, A.S.; Midgley, G.F.; Miles, L.; Ortega-Huerta, M.A.; Peterson, A.T.; Phillips, O.L.; Williams, S.E. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [Green Version]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischmann, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- National Assessment Synthesis Team, Climate Change Impacts on the United States: The Potential Consequences of Climate Variability and Change; U.S. Global Change Research Program: Washington, DC, USA, 2000.
- Snodgrass, J.W.; Bryan, A.L., Jr.; Burger, J. Development of expectations of larval amphibian assemblage structure in southeastern depression wetlands. Ecol. Appl. 2000, 10, 1219–1229. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Mata, L.J.; Arnell, N.W.; Döll, P.; Kabat, P.; Jiménez, B.; Miller, K.A.; Oki, T.; Sen, Z.; Shiklomanov, I.A. Freshwater resources and their management. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van derLinden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 173–210. [Google Scholar]
- Seneviratne, S.I.; Nicholls, N.; Easterling, D.; Goodess, C.M.; Kanae, S.; Kossin, J.; Luo, Y.; Marengo, J.; McInnes, K.; Rahimi, M.; Reichstein, M.; Sorteberg, A.; Vera, C.; Zhang, X. Changes in climate extremes and their impacts on the natural physical environment. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; Field, C.B., Barros, V., Stocker, T.F., Qin, D., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.-K., Allen, S.K., Tignor, M., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, and New York, NY, USA, 2012; pp. 109–230, A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC). [Google Scholar]
- Burke, E.J.; Brown, S.J.; Christidis, N. Modelling the recent evolution of global drought and projections for the 21st century with the Hadley Centre climate model. J. Hydrometeorol. 2006, 7, 1113–1125. [Google Scholar] [CrossRef]
- Greenville, A.C.; Wardle, G.M.; Dickman, C.R. Extreme climatic events drive mammal irruptions: regression analysis of 100-year trends in desert rainfall and temperature. Ecol. Evol. 2012, 2, 2645–2658. [Google Scholar] [CrossRef]
- Schoener, T.W.; Spiller, D.A. Nonsynchronous recovery of community characteristics in island spiders after a catastrophic hurricane. P. Natl. Acad. Sci. USA 2006, 103, 2220–2225. [Google Scholar] [CrossRef]
- Thibault, K.M.; Brown, J.H. Impact of an extreme climatic event on community assembly. P. Natl. Acad. Sci. USA 2008, 105, 3410–3415. [Google Scholar] [CrossRef]
- Scheele, B.C.; Driscoll, D.A.; Fischer, J.; Hunter, D.A. Decline of an endangered amphibian during an extreme climatic event. Ecosphere 2012, 3. [Google Scholar] [CrossRef]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; Ron, S.R.; Sánchez-Azofeifa, G.A.; Still, C.J.; Young, B.E. Widespread amphibian declines from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar]
- Whitfield, S.M.; Bell, K.E.; Phillippi, T.; Sasa, M.; Bolaños, F.; Chaves, G.; Savage, J.M.; Donnelly, M.A. Amphibian and reptile declines over 35 years at La Selva, Costa Rica. P. Natl. Acad. Sci.USA 2007, 104, 8352–8356. [Google Scholar] [CrossRef]
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; Gadsden, H.; Avila, L.J.; Morando, M.; De la Riva, I.J.; Sepulveda, P.V.; Rocha, C.F.D.; Ibargüengoytía, N.; Puntriano, C.A.; Massot, M.; Lepetz, V.; Oksanen, T.A.; Chapple, D.G.; Bauer, A.M.; Branch, W.R.; Clobert, J.; Sites, J.W., Jr. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef]
- Burrowes, P.A.; Joglar, R.L.; Green, D.E. Potential causes for amphibian declines in Puerto Rico. Herpetologica 2004, 60, 141–154. [Google Scholar] [CrossRef]
- Lips, K.R.; Diffendorfer, J.; Mendelson, J.R., III; Sears, M.W. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008, 6, e72. [Google Scholar] [CrossRef]
- Daszak, P.; Scott, D.E.; Kilpatrick, A.M.; Faggioni, C.; Gibbons, J.W.; Porter, D. Amphibian population declines at Savannah River Site are linked to climate, not chytridiomycosis. Ecology 2005, 86, 3232–3237. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Janzen, F.J. Temperature-dependent sex determination and contemporary climate change. Sex. Dev. 2010, 4, 129–140. [Google Scholar] [CrossRef]
- Terhivuo, J. Phenology of spawning for the Common Frog (Rana temporaria L.) in Finland from 1846 to 1986. Ann. Zool. Fenn. 1988, 25, 165–175. [Google Scholar]
- Beebee, T.J.C. Amphibian breeding and climate. Nature 1995, 374, 219–220. [Google Scholar] [CrossRef]
- Gibbs, J.P.; Breisch, A.R. Climate warming and calling phenology of frogs near Ithaca, New York, 1900-1999. Conserv. Biol. 2001, 15, 1175–1178. [Google Scholar] [CrossRef]
- Chadwick, E.A.; Slater, F.M.; Ormerod, S.J. Inter- and intraspecific differences in climatically mediated phenological change in coexisting Triturus species. Glob. Change Biol. 2006, 12, 1069–1078. [Google Scholar] [CrossRef]
- Parmesan, C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 2007, 13, 1860–1872. [Google Scholar] [CrossRef]
- Seimon, T.A.; Seimon, A.; Daszak, P.; Halloy, S.R.P.; Schloegel, L.M.; Aguilar, C.A.; Sowell, P.; Hyatt, A.D.; Konecky, B.; Simmons, J.E. Upward range extension of Andean anurans and chytridiomycosis to extreme elevations in response to tropical deglaciation. Glob. Change Biol. 2007, 13, 288–299. [Google Scholar] [CrossRef]
- Kusano, T.; Inoue, M. Long-term trends toward earlier breeding of Japanense amphibians. J. Herpetol. 2008, 42, 608–614. [Google Scholar] [CrossRef]
- Carroll, E.A.; Sparks, T.H.; Collinson, N.; Beebee, T.J.C. Influence of temperature on the spatial distribution of first spawning dates of the common frog (Rana temporaria) in the UK. Glob. Change Biol. 2009, 15, 467–473. [Google Scholar] [CrossRef]
- Phillimore, A.B.; Hadfield, J.D.; Jones, O.R.; Smithers, R.J. Differences in spawning date between populations of common frog reveal local adaptation. P. Natl. Acad. Sci. USA 2010, 107, 8292–8297. [Google Scholar]
- Todd, B.D.; Scott, D.E.; Pechmann, J.H.K.; Gibbons, J.W. Climate change correlates with rapid delays and advancements in reproductive timing in an amphibian community. Proc. R. Soc. B 2011, 278, 2191–2197. [Google Scholar] [CrossRef]
- Walpole, A.A.; Bowman, J.; Tozer, D.C.; Badzinski, D.S. Community-level response to climate change: shifts in anuran calling phenology. Herpetol. Conserv. 2012, 7, 249–257. [Google Scholar]
- Arnfield, H.; Grant, R.; Monk, C.; Uller, T. Factors influencing the timing of spring migration in common toads (Bufo bufo). J. Zool. 2012, 288, 112–118. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Belden, L.K.; Olson, D.H.; Green, D.M.; Root, T.L.; Kiesecker, J.M. Amphibian breeding and climate change. Conserv. Biol. 2001, 15, 1804–1809. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Sparks, T.; Rybacki, M.; Berger, L. Is body size of the water frog Rana esculenta complex responding to climate change? Naturwissenschaften 2006, 93, 110–113. [Google Scholar] [CrossRef]
- Reading, C.J. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia 2007, 151, 125–131. [Google Scholar] [CrossRef]
- Moreno-Rueda, G.; Pleguezuelos, J.M.; Pizarro, M.; Montori, A. Northward shifts of the distributions of Spanish reptiles in association with climate change. Conserv. Biol. 2012, 26, 278–283. [Google Scholar] [CrossRef]
- Raxworthy, C.J.; Pearson, R.G.; Rabibisoa, N.; Rakotondrazafy, A.M.; Ramanamanjato, J.; Raselimanana, A.P.; Wu, S.; Nussbaum, R.A.; Stone, D.A. Extinction vulnerability of tropical montane endemism from warming and upslope displacement: a preliminary appraisal for the highest massif in Madagascar. Glob. Change Biol. 2008, 14, 1–18. [Google Scholar]
- Buggs, R.J.A. Empirical study of hybrid zone movement. Heredity 2007, 99, 301–312. [Google Scholar] [CrossRef]
- Walls, S.C. The role of climate in the dynamics of a hybrid zone in Appalachian salamanders. Glob. Change Biol. 2009, 15, 1903–1910. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. Population structure, body mass, activity, and orientation of an aquatic snake (Seminatrix pygaea) during a drought. Can. J. Zool. 1993, 71, 1281–1288. [Google Scholar] [CrossRef]
- Willson, J.D.; Winne, C.T.; Dorcas, M.E.; Gibbons, J.W. Post-drought responses of semi-aquatic snakes inhabiting an isolated wetland: insights on different strategies for persistence in a dynamic habitat. Wetlands 2006, 26, 1071–1078. [Google Scholar] [CrossRef]
- Dodd, D.K., Jr.; Dreslik, M.J. Habitat disturbances differentially affect individual growth rates in a long-lived turtle. J. Zool. 2008, 275, 18–25. [Google Scholar] [CrossRef]
- Buhlmann, K.A.; Congdon, J.D.; Gibbons, J.W.; Greene, J.L. Ecology of chicken turtles (Deirochelys reticularia) in a seasonal wetland ecosystem: Exploiting resource and refuge environments. Herpetologica 2009, 65, 39–53. [Google Scholar] [CrossRef]
- Winne, C.T.; Willson, J.D.; Gibbons, J.W. Drought survival and reproduction impose contrasting selection pressures on maximum body size and sexual size dimorphism in a snake, Seminatrix pygaea. Oecologia 2010, 162, 913–922. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr.; Hyslop, N.L.; Oli, M.K. The effects of disturbance events on abundance and sex ratios of a terrestrial turtle, Terrapene bauri. Chelon. Conserv. Biol. 2012, 11, 44–49. [Google Scholar] [CrossRef]
- Yagi, K.T.; Litzgus, J.D. The effects of flooding on the spatial ecology of spotted turtles (Clemmys guttata) in a partially mined peatland. Copeia 2012, 1, 179–190. [Google Scholar] [CrossRef]
- Usuda, H.; Morita, T.; Hasegawa, M. Impacts of river alteration for flood control on freshwater turtle populations. Landscape Ecol. Eng. 2012, 8, 9–16. [Google Scholar] [CrossRef]
- Selman, W.; Qualls, C. The impacts of Hurricane Katrina on a population of yellow-blotched sawbacks (Graptemys flavimaculata) in the Lower Pascagoula River. Herpetol. Conserv. Biol. 2008, 3, 224–230. [Google Scholar]
- Cash, W.B.; Holberton, R.L. Endocrine and behavioral response to a decline in habitat quality: effects of pond drying on the slider turtle, Trachemys scripta. J. Exp. Zool. 2005, 303A, 872–879. [Google Scholar] [CrossRef]
- Lindeman, P.V.; Rabe, F.W. Effect of drought on the western painted turtle, Chrysemys picta belli, in a small wetland ecosystem. J. Freshwater Ecol. 1990, 5, 359–364. [Google Scholar] [CrossRef]
- Sexton, O.J.; Drda, W.J.; Sexon, K.G.; Bramble, J.E. The effects of flooding upon the snake fauna of an isolated refuge. Nat. Area. J. 2007, 27, 133–144. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behavior of Amphibians; The University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Jaeger, R.G. Moisture as a factor influencing the distributions of two species of terrestrial salamanders. Oecologia 1971, 6, 191–207. [Google Scholar] [CrossRef]
- Jaeger, R.G. Density-dependent and density-independent causes of extinction of a salamander population. Evolution 1980, 34, 617–621. [Google Scholar] [CrossRef]
- Stewart, M.M. Climate driven population fluctuations in rain forest frogs. J. Herpetol. 1995, 29, 437–446. [Google Scholar] [CrossRef]
- Brooks, R.T. Weather-related effects on woodland vernal pool hydrology and hydroperiod. Wetlands 2004, 24, 104–114. [Google Scholar] [CrossRef]
- Lake, P.S. Ecological effects of perturbation by drought in flowing waters. Freshwater Biol. 2003, 48, 1161–1172. [Google Scholar] [CrossRef]
- Brooks, R.T. Potential impacts of global climate change on the hydrology and ecology of ephemeral freshwater systems of the forests of the northeastern United States. Clim. Change 2009, 95, 469–483. [Google Scholar] [CrossRef]
- Rodenhouse, N.L.; Christenson, L.M.; Parry, D.; Green, L.E. Climate change effects on native fauna of northeastern forests. Can. J. For. Res. 2009, 39, 249–263. [Google Scholar] [CrossRef]
- Jansen, M.; Schulze, A.; Werding, L.; Streit, B. Effects of extreme drought in the dry season on an anuran community in the Bolivian Chiquitano region. Salamandra 2009, 45, 233–238. [Google Scholar]
- Semlitsch, R.D. Relationship of pond drying to the reproductive success of the salamander Ambystoma talpoideum. Copeia 1987, 1, 61–69. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. Cost of living in an unpredictable environment: the ecology of striped newts Notophthalmus perstriatus during a prolonged drought. Copeia 1993, 3, 605–614. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. The effects of drought on population structure, activity, and orientation of toads (Bufo quercicus and B. terrestris) at a temporary pond. Ethol. Ecol. Evol. 1994, 6, 331–349. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. The ecology of a sandhills population of the eastern narrow-mouthed toad, Gastrophyrne carolinensis, during a drought. Bull. Fl. Mus. Nat. Hist. 1995, 38, 11–41. [Google Scholar]
- Richter, S.C.; Young, J.E.; Johnson, G.N.; Seigel, R.A. Stochastic variation in reproductive success of a rare frog, Rana sevosa: implications for conservation and for monitoring amphibian populations. Biol. Conserv. 2003, 111, 171–177. [Google Scholar] [CrossRef]
- Palis, J.G.; Aresco, M.J.; Kilpatrick, S. Breeding biology of a Florida population of Ambystoma cingulatum (Flatwoods salamander) during a drought. Southeast. Nat. 2006, 5, 1–8. [Google Scholar]
- Taylor, B.E.; Scott, D.E.; Gibbons, J.W. Catastrophic reproductive failure, terrestrial survival, and persistence of the marbled salamander. Conserv. Biol. 2006, 20, 792–801. [Google Scholar] [CrossRef]
- McMenamin, S.K.; Hadly, E.A. Developmental dynamics of Ambystoma tigrinum in a changing landscape. BMC Ecology 2010, 10, 10. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Scott, D.E.; Pechmann, J.H.K. Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 1988, 69, 184–192. [Google Scholar] [CrossRef]
- Kinkead, K.E.; Otis, D.L. Estimating superpopulation size and annual probability of breeding for pond-breeding salamanders. Herpetologica 2007, 63, 151–162. [Google Scholar] [CrossRef]
- Church, D.R.; Bailey, L.L.; Wilbur, H.M.; Kendall, W.L.; Hines, J.E. Iteroparity in the variable environment of the salamander Ambystoma tigrinum. Ecology 2007, 88, 891–903. [Google Scholar] [CrossRef]
- Trauth, J.B.; Trauth, S.E.; Johnson, R.L. Best management practices and drought combine to silence the Illinois chorus frog in Arkansas. Wild. Soc. Bull. 2006, 34, 514–518. [Google Scholar] [CrossRef]
- Werner, E.E.; Relyea, R.A.; Yurewicz, K.L.; Skelly, D.K.; Davis, C.J. Comparative landscape dynamics of two anuran species: climate-driven interaction of local and regional processes. Ecol. Monogr. 2009, 79, 503–521. [Google Scholar] [CrossRef]
- Donald, D.B.; Aitken, W.T.; Paquette, C.; Wulff, S.S. Winter snowfall determines the occupancy of northern prairie wetlands by tadpoles of the Wood Frog (Lithobates sylvaticus). Can. J. Zool. 2011, 89, 1063–1073. [Google Scholar] [CrossRef]
- Price, S.J.; Browne, R.A.; Dorcas, M.E. Resistance and resilience of a stream salamander to supraseasonal drought. Herpetologica 2012, 68, 312–323. [Google Scholar] [CrossRef]
- Lowe, W.H. Climate change is linked to long-term decline in a stream salamander. Biol. Conserv. 2012, 145, 48–53. [Google Scholar] [CrossRef]
- Barrett, K.; Helms, B.S.; Guyer, C.; Schoonover, J.E. Linking process to pattern: causes of stream-breeding amphibian decline in urbanized watersheds. Biol. Conserv. 2010, 143, 1998–2005. [Google Scholar] [CrossRef]
- Cover, M.R.; de la Fuente, J.A.; Resh, V.H. Catastrophic disturbances in headwater streams: the long-term ecological effects of debris flows and debris floods in the Klamath Mountains, northern California. Can. J. Fish. Aquat. Sci. 2010, 67, 1596–1610. [Google Scholar] [CrossRef]
- Kupferberg, S.J.; Palen, W.J.; Lind, A.J.; Bobzien, S.; Catenazzi, A.; Drennan, J.; Power, M.E. Effects of flow regimes altered by dams on survival, population declines, and range-wide losses of California river-breeding frogs. Conserv. Biol. 2012, 26, 513–524. [Google Scholar] [CrossRef]
- Nickerson, M.A.; Pitt, A.L.; Prysby, M.D. The effects of flooding on Hellbender salamander, Cryptobranchus alleganiensis Daudin, 1803, populations. Salamandra 2007, 43, 111–118. [Google Scholar]
- Wojnowski, D. Hurricane Floyd’s effect on the nesting success of the marbled salamander (Ambystoma opacum) at Falls Lake, North Carolina. J. Elisha Mitch. Sci. Soc. 2000, 116, 171–175. [Google Scholar]
- Petranka, J.W. Salamanders of the United States and Canada; Smithsonian Institution Press: Washington, DC, USA, 1998. [Google Scholar]
- Walls, S.C. Personal communication. U.S. Geological Survey: Gainesville, FL, USA, 2013. [Google Scholar]
- Schoener, T.W.; Spiller, D.A.; Losos, J.B. Variable ecological effects of hurricanes: the importance of seasonal timing for survival of lizards on Bahamian islands. P. Natl. Acad. Sci. USA 2004, 101, 177–181. [Google Scholar] [CrossRef]
- Woolbright, L.L. The impact of Hurricane Hugo on forest frogs in Puerto Rico. Biotropica 1991, 23, 462–467. [Google Scholar] [CrossRef]
- Woolbright, L.L. Disturbance influences long-term population patterns in the Puerto Rican frog, Eleutherodactylus coqui (Anura: Leptodactylidae). Biotropica 1996, 28, 493–501. [Google Scholar] [CrossRef]
- Vilella, F.J.; Fogarty, J.H. Diversity and abundance of forest frogs (Anura: Leptodactylidae) before and after Hurricane Georges in the Cordillera Central of Puerto Rico. Caribb. J. Sci. 2005, 41, 157–162. [Google Scholar]
- Schriever, T.A.; Ramspott, J.; Crother, B.I.; Fontenot, C.L., Jr. Effects of Hurricanes Ivan, Katrina, and Rita on a southeastern Louisiana herpetofauna. Wetlands 2009, 29, 112–122. [Google Scholar] [CrossRef]
- Gunzburger, M.S.; Hughes, W.B.; Barichivich, W.J.; Staiger, J.S. Hurricane storm surge and amphibian communities in coastal wetlands of northwestern Florida. Wetl. Ecol. Manag. 2010, 18, 651–663. [Google Scholar] [CrossRef]
- Christman, S.P. Geographic variation for salt water tolerance in the frog Rana sphenocephala. Copeia 1974, 3, 773–778. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Tejedo, M. Local adaptation of an anuran amphibian to osmotically stressful environments. Evolution 2003, 57, 1889–1899. [Google Scholar]
- Brown, M.E.; Walls, S.C. Variation in salinity tolerance among larval anurans: Implications for community composition and the spread of an invasive, non-native species. Copeia 2013, in press. [Google Scholar]
- Luja, V.H.; Rodríguez-Estrella, R. Are tropical cyclones sources of natural selection? Observations on the abundance and behavior of frogs affected by extreme climatic events in the Baja California, Peninsula, Mexico. J. Arid Environ. 2010, 74, 1345–1347. [Google Scholar] [CrossRef]
- Hoffman, A.A.; Sgrò, C.M. Climate change and evolutionary adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef]
- Davis, M.B.; Shaw, R.G.; Etterson, J.R. Evolutionary responses to changing climate. Ecology 2005, 86, 1704–1714. [Google Scholar] [CrossRef]
- Skelly, D.K.; Joseph, L.N.; Possingham, H.P.; Freidenburg, L.K.; Farrugia, T.J.; Kinnison, M.T.; Hendry, A.P. Evolutionary responses to climate change. Conserv. Biol. 2007, 21, 1353–1355. [Google Scholar] [CrossRef]
- Visser, M.E. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 2008, 275, 649–659. [Google Scholar] [CrossRef]
- Chown, S.L.; Hoffmann, A.A.; Kristensen, T.N.; Angilletta, M.J., Jr.; Stenseth, N.C.; Pertoldi, C. Adapting to climate change: A perspective from evolutionary physiology. Clim. Res. 2010, 43, 3–15. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Analysis of climatic factors influencing migrations of the salamander Ambystoma talpoideum. Copeia 1985, 2, 477–489. [Google Scholar] [CrossRef]
- Pechmann, J.H.K.; Scott, D.E.; Semlitsch, R.D.; Caldwell, J.P.; Vitt, L.J.; Gibbons, J.W. Declining amphibian populations: the problem of separating human impacts from natural fluctuations. Science 1991, 253, 892–895. [Google Scholar]
- Semlitsch, R.D.; Scott, D.E.; Pechmann, J.H K.; D Gibbons, J.W. Structure and dynamics of an amphibian community: Evidence from a 16-year study of a natural pond. In Long-Term Studies of Vertebrate Communities; Cody, M.L., Smallwood, J.A., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 217–248. [Google Scholar]
- Todd, B. D.; Winne, C.T. Ontogenetic and interspecific variation in timing of movement and responses to climatic factors during migrations by pond-breeding amphibians. Can. J. Zool. 2006, 84, 715–722. [Google Scholar] [CrossRef]
- Saenz, D.; Fitzgerald, L.A.; Baum, K.A.; Conner, R.N. Abiotic correlates of anuran calling phenology: the importance of rain, temperature, and season. Herpetol. Monogr. 2006, 20, 64–82. [Google Scholar] [CrossRef]
- Karl, T.R.; Knight, R.W. Secular trends of precipitation amount, frequency, and intensity in the United States. B. Am. Meteorol. Soc. 1998, 79, 231–241. [Google Scholar] [CrossRef]
- Keim, B.D. Preliminary analysis of the temporal patterns of heavy rainfall across the southeastern United States. Prof. Geogr. 1997, 49, 94–104. [Google Scholar] [CrossRef]
- Burkett, V.; Kusler, J. Climate change: potential impacts and interactions in wetlands of the United States. J. Am. Water Res. Assoc. 2000, 36, 313–320. [Google Scholar] [CrossRef]
- Heisler-White, J.L.; Knapp, A.K.; Kelly, E.F. Increasing precipitation event size increases above ground net primary productivity in a semi-arid grassland. Oecologia 2008, 158, 129–140. [Google Scholar] [CrossRef]
- Lucas, R.W.; Forseth, I.N.; Casper, B.B. Using rainout shelters to evaluate climate change effects on the demography of Cryptantha flava. J. Ecol. 2008, 96, 514–522. [Google Scholar] [CrossRef]
- Cayuela, H.; Besnard, A.; Béchet, A.; Devictor, V.; Olivier, A. Reproductive dynamics of three amphibian species in Mediterranean wetlands: the role of local precipitation and hydrological regimes. Freshwater Biol. 2012, 57, 2629–2640. [Google Scholar] [CrossRef]
- Touchon, J.C. A treefrog with reproductive mode plasticity reveals a changing balance of selection for nonaquatic egg laying. Am. Nat. 2012, 180, 733–743. [Google Scholar] [CrossRef]
- Griffiths, R.A.; Sewell, D.; McCrea, R.S. Dynamics of a declining amphibian metapopulation: survival, dispersal and the impact of climate. Biol. Conserv. 2010, 143, 485–491. [Google Scholar] [CrossRef]
- Marsh, D.M.; Trenham, P.C. 2001. Metapopulation dynamics and amphibian conservation. Conserv. Biol. 2001, 15, 40–49. [Google Scholar]
- Spear, S.F.; Peterson, C.R.; Matocq, M.D.; Storfer, A. Landscape genetics of the blotched tiger salamander (Ambystoma tigrinum melanostictum). Mol. Ecol. 2005, 14, 2553–2564. [Google Scholar] [CrossRef]
- Greenwald, K.R.; Purrenhage, J.L.; Savage, W.K. Landcover predicts isolation in Ambystoma salamanders across region and species. Biol. Conserv. 2009, 142, 2493–2500. [Google Scholar] [CrossRef]
- Cosentino, B.J.; Phillips, C.A.; Schooley, R.L.; Lowe, W.H.; Douglas, M.R. Linking extinction—colonization dynamics to genetic structure in a salamander metapopulation. Proc. R. Soc. B 2012, 279, 1575–1582. [Google Scholar] [CrossRef]
- Trenham, P.C. Cautious optimism for applied conservation genetics and metapopulation viability analysis. Anim. Conserv. 2010, 13, 123–124. [Google Scholar] [CrossRef]
- Greenwald, K.R. Genetic data in population viability analysis: case studies with ambystomatid salamanders. Anim.Conserv. 2010, 13, 115–122. [Google Scholar] [CrossRef]
- Kinkead, K.E.; Abbott, A.G.; Otis, D.L. Genetic variation among Ambystoma breeding populations on the Savannah River Site. Conserv. Genet. 2007, 8, 281–292. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Semlitsch, R.D. Guide to the Reptiles and Amphibians of the Savannah River Site; Univ. Georgia Press: Athens, GA, USA, 1991. [Google Scholar]
- Patterson, K.K. Life history aspects of paedogenic populations of the mole salamander, Ambystoma talpoideum. Copeia 1978, 4, 649–655. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Harris, R.N.; Wilbur, H.M. Paedomorphosis in Ambystoma talpoideum: maintenance of population variation and alternative life history pathways. Evolution 1990, 44, 1604–1613. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Royle, J.A.; Langtimm, C.A. Estimating site occupancy rates when detection probabilities are less than one. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- Walls, S.C.; Barichivich, W.J.; Brown, M.E.; Scott, D.E.; Hossack, B.R. Influence of drought on salamander occupancy of isolated wetlands on the southeastern Coastal Plain of the United States. Wetlands 2013, in press. [Google Scholar]
- Karl, T.R.; Melillo, J.M.; Peterson, T.C. Global Climate Change Impacts in the United States; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Wilbur, H.M. Complex life cycles. Ann. Rev. Ecol. Syst. 1980, 11, 67–93. [Google Scholar]
- Gamble, L.R.; McGarigal, K.; Compton, B.W. Fidelity and dispersal in the pond-breeding amphibian, Ambystoma opacum: Implications for spatio-temporal population dynamics and conservation. Biol. Conserv. 2007, 139, 247–257. [Google Scholar] [CrossRef]
- Babbitt, K.J.; Tanner, G.W. Use of temporary wetlands by anurans in a hydrologically modified landscape. Wetlands 2000, 20, 313–322. [Google Scholar] [CrossRef]
- Babbitt, K.J.; Baber, M.J.; Tarr, T.L. Patterns of larval amphibian distribution along a wetland hydroperiod gradient. Can. J. Zool. 2003, 81, 1539–1552. [Google Scholar] [CrossRef]
- Werner, E.E.; Skelly, D.K.; Relyea, R.A.; Yurewicz, K.L. Amphibian species richness across environmental gradients. Oikos 2007, 116, 1697–1712. [Google Scholar] [CrossRef]
- Petranka, J.W. Evolution of complex life cycles of amphibians: bridging the gap between metapopulation dynamics and life history evolution. Evol. Ecol. 2007, 21, 751–764. [Google Scholar] [CrossRef]
- Blaustein, R.J. Biodiversity hotspot: the Florida panhandle. BioScience 2008, 58, 784–790. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. Imperiled amphibians: a historical perspective. In Aquatic Fauna in Peril: The Southeastern Perspective; Benz, G.W., Collins, D.E., Eds.; Special Publication 1, Southeast Aquatic Research Institute, Lenz Design & Communications: Decatur, GA, USA, 1997; pp. 165–200. [Google Scholar]
- Comer, P.; Goodin, K.; Tomaino, A.; Hammerson, G.; Kittel, G.; Menard, S.; Nordman, C.; Pyne, M.; Reid, M.; Sneddon, L.; Snow, K. Biodiversity values of geographically isolated wetlands in the United States; NatureServe: Arlington, VA, USA, 2005. [Google Scholar]
- Hanson, C.; Yonavjak, L.; Clarke, C.; Minnemeyer, S.; Boisrobert, L.; Leach, A.; Schleeweis, K. Southern Forests for the Future; World Resources Institute: Washington, DC, USA, 2010. [Google Scholar]
- Center for Biological Diversity, Petition to List 404 Aquatic, Riparian and Wetland Species from the Southeastern United States as Threatened or Endangered under the Endangered Species Act; Center for Biological Diversity: Tucson, AZ, USA, 2010.
- Milanovich, J.R.; Peterman, W.E.; Nibbelink, N.P.; Maerz, J.C. Projected loss of a salamander diversity hotspot as a consequence of projected global climate change. PLoS Biol. 2010, 5, e12189. [Google Scholar]
- Wilbur, H.M. Coping with chaos: toads in ephemeral ponds. Trends Ecol. Evol. 1990, 5, 37. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Walls, S.C.; Barichivich, W.J.; Brown, M.E. Drought, Deluge and Declines: The Impact of Precipitation Extremes on Amphibians in a Changing Climate. Biology 2013, 2, 399-418. https://doi.org/10.3390/biology2010399
Walls SC, Barichivich WJ, Brown ME. Drought, Deluge and Declines: The Impact of Precipitation Extremes on Amphibians in a Changing Climate. Biology. 2013; 2(1):399-418. https://doi.org/10.3390/biology2010399
Chicago/Turabian StyleWalls, Susan C., William J. Barichivich, and Mary E. Brown. 2013. "Drought, Deluge and Declines: The Impact of Precipitation Extremes on Amphibians in a Changing Climate" Biology 2, no. 1: 399-418. https://doi.org/10.3390/biology2010399
APA StyleWalls, S. C., Barichivich, W. J., & Brown, M. E. (2013). Drought, Deluge and Declines: The Impact of Precipitation Extremes on Amphibians in a Changing Climate. Biology, 2(1), 399-418. https://doi.org/10.3390/biology2010399