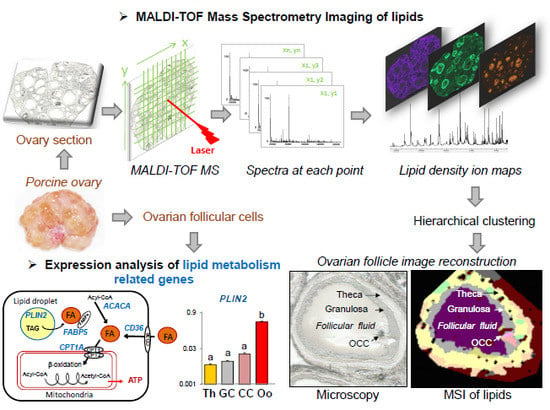
MALDI Mass Spectrometry Imaging of Lipids and Gene Expression Reveals Differences in Fatty Acid Metabolism between Follicular Compartments in Porcine Ovaries
Abstract
:1. Introduction
2. Experimental Section
2.1. Ethics
2.2. Reagents
2.3. Tissue Collection and Preparation
2.4. Matrix Coating and MALDI MSI

2.5. MALDI MSI Lipid Profiling Data Analysis
2.6. RNA Analysis: Quantification of Gene Expression
| Gene | Primer | Séquence 5'–3' | Accession Number | Amplicon (bp) | Gene Product | Primers Efficiency |
|---|---|---|---|---|---|---|
| ACACA | fw | TGGAGAAACAGCTGACGGAG | EU168399.1 | 196 | Acetyl coenzyme A carboxylase | |
| rev | GAGAGGATCCGGACGACTTC | 1.99 | ||||
| CD36 | fw | TTGGCCTATGAACCGTTTACT | NM_001044622.1 | 249 | CD36 molecule (thrombospondin receptor) | |
| rev | CGTTCTGAAGTTGCCAAGCA | 1.91 | ||||
| CPTA1 | fw | ACGTGTCGAAAGAAGGAGGT | NM_001129805.1 | 138 | Carnitine palmitoyl transferase 1 A | |
| rev | ACATCCCAAAGAGCATATCGTA | 1.95 | ||||
| FABP5 | fw | ATGGGTGCAATGGCCAAAC | NM_001039746.2 | 221 | Fatty acid binding protein 5 | |
| rev | GAACCACCACCATGGCATAGA | 1.91 | ||||
| PLIN2 | fw | GTGCCACACCCTCGTG | NM_214200.2 | 242 | Perilipin 2 (alias Adipophilin) | 2.00 |
| rev | AGGGACCTACCAGCCAGTT | |||||
| RPL19 | fw | ATGAAATCGCCAACGCCAAC | AF435591.1 | 173 | Ribosomal protein L19 | 1.96 |
| rev | AGCATTGGCAGTACCCTTCC | |||||
| RPS9 | fw | GTGCTGGGGTGCTCTTTAGT | XM_005664825.1 | 160 | Ribosomal protein S9 | 1.92 |
| rev | GAGCCCATATTCGCCGATCA |
3. Results and Discussion
3.1. MALDI MSI Analysis of Porcine Ovarian Sections
3.1.1. Ovarian Lipid Distribution


3.1.2. Intrafollicular Lipid Distribution by MALDI MSI




3.2. Expression of Lipid Metabolism Genes Varied according to Follicular Compartments

4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fair, T. Follicular oocyte growth and acquisition of developmental competence. Anim. Reprod. Sci. 2003, 78, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Collado-Fernandez, E.; Picton, H.M.; Dumollard, R. Metabolism throughout follicle and oocyte development in mammals. Int. J. Dev. Biol. 2012, 56, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Songsasen, N. Energy metabolism regulating mammalian oocyte maturation. In Meiosis—Molecular Mechanisms and Cytogenetic Diversity; Swan, A., Ed.; InTech: Rijeka, Yugoslavia, 2012; pp. 173–186. [Google Scholar]
- Leroy, J.L.; Sturmey, R.G.; van Hoeck, V.; de Bie, J.; McKeegan, P.J.; Bols, P.E. Dietary fat supplementation and the consequences for oocyte and embryo quality: Hype or significant benefit for dairy cow reproduction? Reprod. Domest. Anim. 2014, 49, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.H. Reduced quality and accelerated follicle loss with female reproductive aging—Does decline in theca dehydroepiandrosterone (DHEA) underlie the problem? J. Biomed. Sci. 2013, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010, 139, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; Saraiva, S.A.; Catharino, R.R.; Garcia, J.S.; Gozzo, F.C.; Sanvido, G.B.; Santos, L.F.; lo Turco, E.G.; Pontes, J.H.; Basso, A.C.; et al. Single embryo and oocyte lipid fingerprinting by mass spectrometry. J. Lipid Res. 2010, 51, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Abayasekara, D.R.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Badin, P.M.; Langin, D.; Moro, C. Dynamics of skeletal muscle lipid pools. Trends Endocrinol. Metab. 2013, 24, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Mashek, D.G. Hepatic fatty acid trafficking: Multiple forks in the road. Adv. Nutr. 2013, 4, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lazo, L.; Brisard, D.; Elis, S.; Maillard, V.; Uzbekov, R.; Labas, V.; Desmarchais, A.; Papillier, P.; Monget, P.; Uzbekova, S. Fatty Acid synthesis and oxidation in cumulus cells support oocyte maturation in bovine. Mol. Endocrinol. 2014, 28, 1502–1521. [Google Scholar] [CrossRef] [PubMed]
- Paczkowski, M.; Silva, E.; Schoolcraft, W.B.; Krisher, R.L. Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biol. Reprod. 2013, 88, 111. [Google Scholar] [CrossRef] [PubMed]
- Aardema, H.; Lolicato, F.; van de Lest, C.H.A.; Brouwers, J.F.; Vaandrager, A.B.; van Tol, H.T.A.; Roelen, B.A.J.; Vos, P.L.A.M.; Helms, J.B.; Gadella, B.M. Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intracellular lipid storage. Biol. Reprod. 2013, 88. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, F.; Brouwers, J.F.; de Lest, C.H.; Wubbolts, R.; Aardema, H.; Priore, P.; Roelen, B.A.; Helms, J.B.; Gadella, B.M. The cumulus cell layer protects the bovine maturing oocyte against fatty acid-induced lipotoxicity. Biol. Reprod. 2015, 92, 16. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.; Russell, D.L.; Robker, R. Lipids and oocyte developmental competence: The role of fatty acids and β-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- Homa, S.T.; Racowsky, C.; McGaughey, R.W. Lipid analysis of immature pig oocytes. J. Reprod. Fertil. 1986, 77, 425–434. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 2000, 118, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; Eberlin, L.S.; Hallett, J.E.; Cooks, R.G. Single oocyte and single embryo lipid analysis by desorption electrospray ionization mass spectrometry. J. Mass Spectrom. 2012, 47, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.A.; Cordeiro, F.B.; Regiani, T.; Victorino, A.B.; Pilau, E.J.; Gozzo, F.C.; Ferreira, C.R.; Fraietta, R.; lo Turco, E.G. The follicular microenviroment as a predictor of pregnancy: MALDI-TOF MS lipid profile in cumulus cells. J. Assist. Reprod. Genet. 2012, 29, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Pirro, V.; Oliveri, P.; Ferreira, C.R.; Gonzalez-Serrano, A.F.; Machaty, Z.; Cooks, R.G. Lipid characterization of individual porcine oocytes by dual mode DESI-MS and data fusion. Anal. Chim. Acta 2014, 848, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Prates, E.G.; Alves, S.P.; Marques, C.C.; Baptista, M.C.; Horta, A.E.; Bessa, R.J.; Pereira, R.M. Fatty acid composition of porcine cumulus oocyte complexes (COC) during maturation: Effect of the lipid modulators trans-10, cis-12 conjugated linoleic acid (t10,c12 CLA) and forskolin. In Vitro Cell Dev. Biol. Anim. 2013, 49, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, T.; Cordeiro, F.B.; Costa Ldo, V.; Pilau, E.J.; Ferreira, C.R.; Gozzo, F.C.; Eberlin, M.N.; Bertolla, R.P.; Cedenho, A.P.; Turco, E.G. Lipid profiling of follicular fluid from women undergoing IVF: Young poor ovarian responders versus normal responders. Hum. Fertil. (Camb.) 2013, 16, 269–277. [Google Scholar] [CrossRef]
- Meding, S.; Walch, A. MALDI imaging mass spectrometry for direct tissue analysis. Methods Mol. Biol. 2013, 931, 537–546. [Google Scholar] [PubMed]
- Rompp, A.; Spengler, B. Mass spectrometry imaging with high resolution in mass and space. Histochem. Cell Biol. 2013, 139, 759–783. [Google Scholar] [CrossRef] [PubMed]
- Cimino, J.; Calligaris, D.; Far, J.; Debois, D.; Blacher, S.; Sounni, N.E.; Noel, A.; de Pauw, E. Towards lipidomics of low-abundant species for exploring tumor heterogeneity guided by high-resolution mass spectrometry imaging. Int. J. Mol. Sci. 2013, 14, 24560–24580. [Google Scholar] [CrossRef] [PubMed]
- Schone, C.; Hofler, H.; Walch, A. MALDI imaging mass spectrometry in cancer research: Combining proteomic profiling and histological evaluation. Clin. Biochem. 2013, 46, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Lagarrigue, M.; Lavigne, R.; Guevel, B.; Com, E.; Chaurand, P.; Pineau, C. Matrix-assisted laser desorption/ionization imaging mass spectrometry: A promising technique for reproductive research. Biol. Reprod. 2012, 86, 74. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.O.; Oehler, M.K.; Ruszkiewicz, A.; McColl, S.R.; Hoffmann, P. MALDI Imaging Mass Spectrometry (MALDI-IMS)-application of spatial proteomics for ovarian cancer classification and diagnosis. Int. J. Mol. Sci. 2011, 12, 773–794. [Google Scholar] [CrossRef] [PubMed]
- Valckx, S.D.; van Hoeck, V.; Arias-Alvarez, M.; Maillo, V.; Lopez-Cardona, A.P.; Gutierrez-Adan, A.; Berth, M.; Cortvrindt, R.; Bols, P.E.; Leroy, J.L. Elevated non-esterified fatty acid concentrations during in vitro murine follicle growth alter follicular physiology and reduce oocyte developmental competence. Fertil. Steril. 2014, 102. [Google Scholar] [CrossRef] [PubMed]
- Valckx, S.D.; Arias-Alvarez, M.; de Pauw, I.; Fievez, V.; Vlaeminck, B.; Fransen, E.; Bols, P.E.; Leroy, J.L. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: A descriptive cross-sectional study. Reprod. Biol. Endocrinol. 2014, 12, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genicot, G.; Leroy, J.; Soom, A.; Donnay, I. The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology 2005, 63, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Elis, S.; Coyral-Castel, S.; Freret, S.; Cognie, J.; Desmarchais, A.; Fatet, A.; Rame, C.; Briant, E.; Maillard, V.; Dupont, J. Expression of adipokine and lipid metabolism genes in adipose tissue of dairy cows differing in a female fertility quantitative trait locus. J. Dairy Sci. 2013, 96, 7591–7602. [Google Scholar] [CrossRef] [PubMed]
- Meriaux, C.; Franck, J.; Wisztorski, M.; Salzet, M.; Fournier, I. Liquid ionic matrixes for MALDI mass spectrometry imaging of lipids. J. Proteomics 2010, 73, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tsutsumi, K.; Sugiura, Y.; Setou, M. Medical molecular morphology with imaging mass spectrometry. Med. Mol. Morphol. 2009, 42, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Shimma, S.; Sugiura, Y.; Hayasaka, T.; Hoshikawa, Y.; Noda, T.; Setou, M. MALDI-based imaging mass spectrometry revealed abnormal distribution of phospholipids in colon cancer liver metastasis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 855, 98–103. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Y.; Zhou, D.; Li, Z. Significantly increased monounsaturated lipids relative to polyunsaturated lipids in six types of cancer microenvironment are observed by mass spectrometry imaging. Sci. Rep. 2014, 4, 5959. [Google Scholar] [PubMed]
- Leroy, J.L.; Vanholder, T.; Delanghe, J.R.; Opsomer, G.; van Soom, A.; Bols, P.E.; de Kruif, A. Metabolite and ionic composition of follicular fluid from different-sized follicles and their relationship to serum concentrations in dairy cows. Anim. Reprod. Sci. 2004, 80, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, E.S.; Macones, G.A.; Odem, R.R.; Patterson, B.W.; Lanzendorf, S.E.; Ratts, V.S.; Moley, K.H. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil. Steril. 2011, 95, 1970–1974. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.; Cillero-Pastor, B.; Eijkel, G.; Bruinen, A.L.; Ruiz-Romero, C.; Heeren, R.M.; Blanco, F.J. Characterization of lipidic markers of chondrogenic differentiation using mass spectrometry imaging. Proteomics 2015, 15, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Apparicio, M.; Ferreira, C.R.; Tata, A.; Santos, V.G.; Alves, A.E.; Mostachio, G.Q.; Pires-Butler, E.A.; Motheo, T.F.; Padilha, L.C.; Pilau, E.J.; et al. Chemical composition of lipids present in cat and dog oocyte by matrix-assisted desorption ionization mass spectrometry (MALDI- MS). Reprod. Domest. Anim. 2012, S47, 113–117. [Google Scholar] [CrossRef]
- Jarkovska, K.; Martinkova, J.; Liskova, L.; Halada, P.; Moos, J.; Rezabek, K.; Gadher, S.J.; Kovarova, H. Proteome mining of human follicular fluid reveals a crucial role of complement cascade and key biological pathways in women undergoing in vitro fertilization. J. Proteome Res. 2010, 9, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Fahiminiya, S.; Gerard, N. Follicular fluid in mammals. Gynecol. Obstet. Fertil. 2010, 38, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Leroy, J.L.; Vanholder, T.; Delanghe, J.R.; Opsomer, G.; van Soom, A.; Bols, P.E.; Dewulf, J.; de Kruif, A. Metabolic changes in follicular fluid of the dominant follicle in high-yielding dairy cows early post partum. Theriogenology 2004, 62, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.; Bao, S.N.; Jivago, J.L.; Lucci, C.M. Ultrastructural characterization of porcine oocytes and adjacent follicular cells during follicle development: Lipid component evolution. Theriogenology 2011, 76, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V., Jr.; Ory, D.S.; Schaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- Yada, H.; Hosokawa, K.; Tajima, K.; Hasegawa, Y.; Kotsuji, F. Role of ovarian theca and granulosa cell interaction in hormone productionand cell growth during the bovine follicular maturation process. Biol. Reprod. 1999, 61, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Tata, A.; Sudano, M.J.; Santos, V.G.; Landim-Alvarenga, F.D.; Ferreira, C.R.; Eberlin, M.N. Optimal single-embryo mass spectrometry fingerprinting. J. Mass. Spectrom. 2013, 48, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, A.; Bevilacqua, C.; Benne, F.; Bodin, L.; Cotinot, C.; Liaubet, L.; Sancristobal, M.; Sarry, J.; Terenina, E.; Martin, P.; et al. Transcriptome profiling of sheep granulosa cells and oocytes during early follicular development obtained by laser capture microdissection. BMC Genomics 2011, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Matravadia, S.; Holloway, G.P.; Wright, D.C. FAT/CD36 regulates PEPCK expression in adipose tissue. Am. J. Physiol. Cell Physiol. 2013, 304, C478–C484. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.L.; Senthivinayagam, S.; Moon, K.C.; Gupta, S.; Lwande, J.S.; Murphy, C.C.; Storey, S.M.; Atshaves, B.P. Direct interaction of Plin2 with lipids on the surface of lipid droplets: A live cell FRET analysis. Am. J. Physiol. Cell Physiol. 2012, 303, C728–C742. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-X.; Xiong, Y.; Guan, K.-L. Nutrient Sensing, Metabolism, and Cell Growth Control. Mol. Cell 2013, 49, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.J.; Sridhar, K.; Bernlohr, D.A.; Kraemer, F.B. Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proc. Natl. Acad. Sci. USA 1999, 96, 5528–5532. [Google Scholar] [CrossRef] [PubMed]
- Chmurzynska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Serrano, A.F.; Pirro, V.; Ferreira, C.R.; Oliveri, P.; Eberlin, L.S.; Heinzmann, J.; Lucas-Hahn, A.; Niemann, H.; Cooks, R.G. Desorption electrospray ionization mass spectrometry reveals lipid metabolism of individual oocytes and embryos. PLOS ONE 2013, 8, e74981. [Google Scholar] [CrossRef] [PubMed]
- Auclair, S.; Uzbekov, R.; Elis, S.; Sanchez, L.; Kireev, I.; Lardic, L.; Dalbies-Tran, R.; Uzbekova, S. Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E599–E613. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Kwong, W.Y.; Li, D.; Salter, A.M.; Lea, R.G.; Sinclair, K.D. Effects of omega-3 and -6 polyunsaturated fatty acids on ovine follicular cell steroidogenesis, embryo development and molecular markers of fatty acid metabolism. Reproduction 2011, 141, 105–118. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzbekova, S.; Elis, S.; Teixeira-Gomes, A.-P.; Desmarchais, A.; Maillard, V.; Labas, V. MALDI Mass Spectrometry Imaging of Lipids and Gene Expression Reveals Differences in Fatty Acid Metabolism between Follicular Compartments in Porcine Ovaries. Biology 2015, 4, 216-236. https://doi.org/10.3390/biology4010216
Uzbekova S, Elis S, Teixeira-Gomes A-P, Desmarchais A, Maillard V, Labas V. MALDI Mass Spectrometry Imaging of Lipids and Gene Expression Reveals Differences in Fatty Acid Metabolism between Follicular Compartments in Porcine Ovaries. Biology. 2015; 4(1):216-236. https://doi.org/10.3390/biology4010216
Chicago/Turabian StyleUzbekova, Svetlana, Sebastien Elis, Ana-Paula Teixeira-Gomes, Alice Desmarchais, Virginie Maillard, and Valerie Labas. 2015. "MALDI Mass Spectrometry Imaging of Lipids and Gene Expression Reveals Differences in Fatty Acid Metabolism between Follicular Compartments in Porcine Ovaries" Biology 4, no. 1: 216-236. https://doi.org/10.3390/biology4010216
APA StyleUzbekova, S., Elis, S., Teixeira-Gomes, A. -P., Desmarchais, A., Maillard, V., & Labas, V. (2015). MALDI Mass Spectrometry Imaging of Lipids and Gene Expression Reveals Differences in Fatty Acid Metabolism between Follicular Compartments in Porcine Ovaries. Biology, 4(1), 216-236. https://doi.org/10.3390/biology4010216