1. Introduction
The main objectives of this study were to characterize the principal dermal components of the dermis according to the depth of acquisition and to perform a comparison with other techniques (High Resolution Ultrasound Scanning and Cutometry) that give information about the structure of the dermis linked to the age of the volunteers.
Multiphoton microscopy is a noninvasive high resolution imaging technique for depth resolved skin analysis. Multiphoton Microscopy (MPM) detects the two-photon excited autofluorescence (AF) and second harmonic generation (SHG). The AF signal is coming from skin endogenuous fluorophores while the SHG signal comes from collagen. The specificity of these signals allows identification of morphological structures of the skin.
For this reason, the use of the multiphoton imaging technique is increasing in applied dermatological research, especially in skin aging studies, particularly to image and quantify the extracellular matrix components, elastin and collagen. The evolution of the SAAID (SHG-to-AF Aging Index of Dermis) index as a function of the depth of the dermis in differently aged population has been reported [
1,
2,
3,
4]. The Fluorescence Lifetime Imaging Measurement (FLIM) technique also demonstrated a correlation of the dermal components (elastin and collagen) with the location and aging [
5].
While the first studies using the multiphoton approach were done on forearms, more recent studies due to evolution of the techniques were conducted on photoaged facial skin where the intensity of the SHG signal and SAAID correlated significantly with aging [
6].
One recent study introduced a different ratio, the elastin-to-collagen ratio (ELCOR) [
7]. The ELCOR parameter on the sun protected volar forearm skin significantly increased with age and sun exposure. ELCOR is another parameter for the characterization of the modifications of extracellular matrix (ECM) components.
Recently, the technique of multiphoton microscopy has been also used for the evaluation of cosmetic treatment on the ECM components [
8].
High frequency ultrasound is a noninvasive tool that can provide information on various cutaneous parameters such as skin thickness, dermal density and age related echogenicity [
9,
10,
11].
The principle consists of using an ultrasound scanner equipped with a 50 MHz transducer (DermCup, Atys Medical, Soucieu en Jarrest, France) allowing recovery of a high resolution image of a cross section of the skin. The resolution in the plane of sections reaches 45 µm for a 6 mm depth and 16 mm width. The echogenicity of the dermis can be quantified at different depths by image analysis, which allows demonstration of a possible low echogenicity of the sub-epidermal part (SENEB: Sub Epidermal Non Echogenic Band). This low echogenicity, already well documented in the literature, is correlated to disorganization of the collagen network, its fragmentation caused by photoexposure and aging of the skin [
12].
The evolution of biomechanical properties with aging is the subject of numerous studies. Many different noninvasive devices using alternative measuring approaches such as stretching, torsion, indentation and suction have been developed for biomechanical properties evaluation. The measurements of skin deformation after suction or torsion are the most widely used techniques in cosmetic research [
13,
14].
The modification of skin elasticity and firmness with aging are largely reported in the bibliography [
15,
16,
17,
18], but to our knowledge, there are no data that would make a link between the evolution of biomechanical properties, skin echogenicity and components of ECM.
2. Materials and Methods
Multiphoton tomography with a femtosecond Ti-Sapphire laser was used to quantify elastin and collagen with SHG-to-AF Aging Index of Dermis (SAAID) (DermaInspect®, Jenlab GmbH, Saarbrücken, Germany). Images of the elastin and collagen fluorescence were acquired simultaneously with 2 photomultiplier tube detectors on the volar forearm in two depths in the dermis: superficial part of dermis and 25 µm deeper. The image size was 300 µm × 300 µm with the resolution of 1 µm in x, y axis and 2 µm in z axis and images were recorded on a 512 × 512 pixel matrix. The excitation wavelength was 805 nm and the use of an appropriate filter allowed the acquisition of the elastin and collagen signals. The laser set up was kept constant for all volunteers.
The quantification of elastin and collagen consisted in evaluation of grey level in the region of interest (ROI) (
Figure 1). The analysis was done with specific software “FrameScan-OrionTechnoLab™” developed by Orion Concept. The values in the tables correspond to the relative intensity on a grey level scale between 0 and 255.
Figure 1.
Definition of region of interest (ROI) for the quantification of collagen (second harmonic generation (SHG), green) and elastin (autofluorescence (AF), red) intensities.
Figure 1.
Definition of region of interest (ROI) for the quantification of collagen (second harmonic generation (SHG), green) and elastin (autofluorescence (AF), red) intensities.
The quantified parameters were:
relative fluorescence intensity of collagen
relative fluorescence intensity of elastin
ratio of intensity for collagen/elastin
SAAID = (Intensity of Collagen – Intensity of Elastin)/(Inten,sity of Collagen + Intensity of Elastin)
In parallel, a 50 MHz ultrasound scanner (Dermcup, Athys Medical, Soucieu en Jarrest, France) was used for the calculation of SENEB. Acquisitions were performed on the same areas as for the multiphoton microscopy and quantification was done by specific software (EchoScan V02, OrionTechnoLab, ORION Concept, Tours, France). On ultrasound images, two equivalent regions of interest were placed on the most superficial and deep third part of the dermis for the calculation of the echogenicity. The “relative SENEB” indicated a calculation of the superficial echogenicity relatively to deeper one.
To validate the difference of the two studied groups in terms of skin photoaging, measurements of the skin mechanical properties and their modifications related to the age were performed with a cutometer on the face with a 2 mm probe (Cutometer® MPA 580, Courage+Khazaka Electronic GmbH, Köln, Germany).
The conditions of measurements were: negative pressure 500 Mbar, the suction of 3 s followed by 5 s of release.
On the basis of the deformation curves (
Figure 2), the parameters
Ue (immediate deformation),
Uf (final deformation),
Ur (immediate retraction) have been determined and used for the calculation of the ratio parameters: net elasticity (
Ur/
Ue), elastic recovery or firmness (
Ur/
Uf) and viscoelastic ratio (
Uv/
Ue).
Figure 2.
Example of the deformation curve with the indications of parameters.
Figure 2.
Example of the deformation curve with the indications of parameters.
The study was done on two groups of 30 healthy female Caucasian volunteers.
All subjects had signed a free participation agreement. This study not involving techniques or invasive processes, it was not necessary to perform a request with the ethics committee, according to the French and European legislation.
The mean age ± SD of the “Young” group was 28 ± 9 years and the mean age of “Aged” group was 54 ± 11 years.
The acquisitions were performed after acclimatization of the volunteers for 30 min in standardized conditions (Temperature 21 ± 2 °C, Relative Humidity 65% ± 5%).
Multiphoton microscopy acquisition as well as High Resolution Ultrasound Scanning were done on the external forearm while the cutometer measurements were done on the face (cheek).
If the measures involving the multiphoton microscope and the ultrasound imaging could not be realized on the face in good conditions of reliability, those of the mechanical properties seemed more relevant for this localization which remains privileged within the framework of application of the skincare products. The configuration of the multiphoton microscope did not allow a comfortable use for acquisitions on the face and it was preferable to couple the measure of echography with the microscopy to allow a good correlation of the imaging and the obtained quantifications. Moreover, the correlation between the age and the modifications of the mechanical properties of the skin with the Cutometer was already widely demonstrated both on the face and on the forearm.
3. Results and Discussion
3.1. Multiphoton Microscopy
The SAAID ratio was 0.11 ± 0.02 for the young group and 0.07 ± 0.02 for the aged group of volunteers in the superior dermis and 0.08 ± 0.02 for the young group and 0.03 ± 0.01 for the aged group of volunteers in the inferior dermis. This decrease represents 36.4% for the young group and 62.5% for the aged groups of volunteers. A decrease of the SAAID ratio was observed with the depth of the dermis for both age groups (
Figure 3).
Figure 3.
SHG-to-AF Aging Index of Dermis (SAAID) modification with depth of dermis and age. Measurements were performed on the volar forearm. The results are shown as mean ± SEM (n = 30) and p values are indicated for the significant difference.
Figure 3.
SHG-to-AF Aging Index of Dermis (SAAID) modification with depth of dermis and age. Measurements were performed on the volar forearm. The results are shown as mean ± SEM (n = 30) and p values are indicated for the significant difference.
The reduction of the SAAID ratio was more pronounced in the inferior dermis and the correlation of SAAID with aging is significant as illustrated in the
Figure 4.
These results corroborate the already published data on the diminution of the SAAID with age [
1,
7]. The diminution of the SAAID between superior and inferior dermis was more pronounced in the older group of volunteers (−57.1%) compared to the young group (−27.3%) which reflects more important modifications of ECM components with age.
The level of collagen and elastin were also changed as a function of the depth of the dermis and age (
Figure 5).
Figure 4.
Evolution of SAAID and elastin with aging in the inferior dermis. Relationship between SAAID, elastin autofluorescence and age. The regression line shows the signals as a function of age. R = Pearson coefficient of correlation, p value indicates the significance level of correlation.
Figure 4.
Evolution of SAAID and elastin with aging in the inferior dermis. Relationship between SAAID, elastin autofluorescence and age. The regression line shows the signals as a function of age. R = Pearson coefficient of correlation, p value indicates the significance level of correlation.
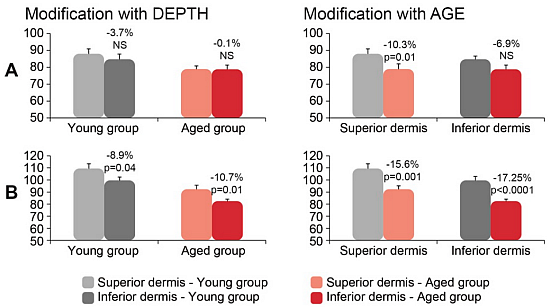
Figure 5.
Collagen (A) and elastin intensity (B) modification with depth of dermis and age. Measurements were performed on the volar forearm. The results are shown as mean ± SEM (n = 30) and p values are indicated for the significant difference.
Figure 5.
Collagen (A) and elastin intensity (B) modification with depth of dermis and age. Measurements were performed on the volar forearm. The results are shown as mean ± SEM (n = 30) and p values are indicated for the significant difference.
Comparison between the two age categories revealed in a superior dermis a significant decrease of the collagen (88.0 ± 2.9 young vs. 78.9 ± 1.9 old) and elastin (109.5 ± 3.9 young vs. 92.4 ± 3.3 aged).
The changes as a function of the depth of dermis are significant only for elastin and that for both groups, young and aged. The lower dermis is less rich in elastin as can be judged from the diminution of the intensity by 8.9% and 10.7% for the young and aged group respectively.
In the inferior dermis a significant difference between the young and old group was obtained for elastin (99.7 ± 2.7 young vs. 82.5 ± 1.7 old).
Some authors have not found a difference in elastin level as a function of age [
6]. Elastin level remained stable in their study and the decline of SAAID was linked only to the lower intensity of collagen. Our results have demonstrated the significant elastin level reduction with age and this correlation was significant in the inferior dermis (
Figure 5).
As to collagen/elastin ratio, in superior dermis this ratio is 0.82 ± 0.03 for the young group and 0.89 ± 0.05 for the aged group, while in the inferior dermis it is 0.86 ± 0.03 for the young group and 0.96 ± 0.03 for the aged group. The collagen/elastin ratio increased with the depth of the dermis by 4.9% for the young group and 7.9% for the aged group.
The increase of collagen/elastin ratio in our work is the consequence of higher decline of elastin with age.
An example of the difference of collagen and elastin level between young and aged skin is illustrated in
Figure 6.
Figure 6.
In vivo optical biopsy illustrating collagen and elastin levels. Collagen (SHG-signal in green) and elastin (autofluorescence in red) distribution in function of age. The serial images from Multiphoton microscopy were reconstructed and visualized with platform SkinExplorer™. R = −0.246; p < 0.0001.
Figure 6.
In vivo optical biopsy illustrating collagen and elastin levels. Collagen (SHG-signal in green) and elastin (autofluorescence in red) distribution in function of age. The serial images from Multiphoton microscopy were reconstructed and visualized with platform SkinExplorer™. R = −0.246; p < 0.0001.
3.2. High Resolution Echography
The considered parameters for echography were: echogenicity of superior and inferior dermis and relative SENEB.
The degradation and the disorganization of the superficial collagen network under the influence of photoaging induce a sensitive decrease of the echogenicity compared with that of the deep dermis (relative SENEB). This parameter gives reliable information about the level of degradation of the superficial dermis in terms of solar elastosis and photoaging.
A good and significant correlation with aging has been found for echogenicity of superior dermis and for SENEB (
Figure 7).
Figure 7.
Evolution of echogenicity and SENEB with aging. The regression line shows the parameters as a function of age. R = Pearson coefficient of correlation, p value indicates the significance level of correlation.
Figure 7.
Evolution of echogenicity and SENEB with aging. The regression line shows the parameters as a function of age. R = Pearson coefficient of correlation, p value indicates the significance level of correlation.
Comparing these parameters between young and aged skin, we observed significant differences between these two groups in echogenicity of the dermis (115.4 ± 4.0 young vs. 97.0 ± 5.5 aged) and relative SENEB (−14.5 ± 2.71 young vs. −41.3 ± 3.85 aged).
3.3. Biomechanical Properties
The evolution of skin biomechanical properties with aging is well known. The observed evolution of the ratio parameters
Ur/
Ue,
Ur/
Uf and
Uv/
Ue is presented in
Figure 8.
Figure 8.
Evolution of skin biomechanical properties with aging. The regression line shows the selected biomechanical parameters as a function of age. R = Pearson coefficient of correlation, p value indicates the significance level of correlation. Percentages indicate the difference of parameter between aged and young group of volunteers (n = 30).
Figure 8.
Evolution of skin biomechanical properties with aging. The regression line shows the selected biomechanical parameters as a function of age. R = Pearson coefficient of correlation, p value indicates the significance level of correlation. Percentages indicate the difference of parameter between aged and young group of volunteers (n = 30).
Relating to the significant correlation of biomechanical parameters as a function of age, the direct comparison allowed confirmation of the significant decrease in skin elasticity
Ur/
Ue (−23%) and skin firmness
Ur/
Uf (−27%). On the contrary, the viscoelastic deformation
Uv/
Ue of the skin increased with age by 25% (
Figure 8).
Our results have confirmed the evolution of biomechanical properties with age already discussed frequently in the literature [
14,
15,
16,
17,
18]. The obtained significant evolution of main ratio parameters with age demonstrates the loss of elasticity and firmness of the skin.
The skin biomechanical properties correlated well with the clinical signs of aging.
The three techniques involved in this study allowed evaluation of the composition (Multiphoton), the structure (Ultrasound scanning) and in consequence, the biomechanical properties of the skin (Cutometry). This approach shows and confirms a real relationship between the clinical degradation of the mechanical properties evaluated by the cutometer and its origin in the modification of the structure of the collagen network quantified by the ultrasound scanning. The mutiphoton confocal microscopy gives an additional and complementary level of understanding by defining the biochemical composition of the dermic fibrous network and its variations related to the skin aging.
Finally, the combination of these three techniques allows a description of the whole process of aging of the dermis, from the nature and the proportion of the fibrous compounds in the dermis (collagen and elastin) until its consequence: the loss of the cutaneous mechanical properties.