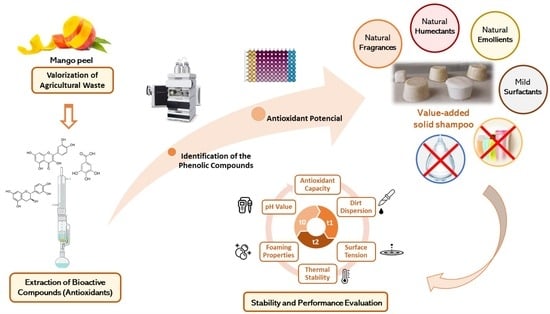
On the Path to Sustainable Cosmetics: Development of a Value-Added Formulation of Solid Shampoo Incorporating Mango Peel Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction Methods
2.2.1. Extraction of Phenolic Compounds from Mango Peel
2.2.2. Essential Oil Extraction from Orange Peel
2.3. Characterization of the Mango Peel Extract
2.3.1. Total Phenolic Content
2.3.2. Antioxidant Capacity
2.3.3. High-Performance Liquid Chromatography (HPLC-DAD)
2.4. Solid Shampoo Production
2.5. Stability and Performance Analysis
2.5.1. Determination of pH
2.5.2. Dirt Dispersion Test
2.5.3. Surface Tension Measurement
2.5.4. Accelerated Thermal Stability Test
2.5.5. Oxidative Stability Test
2.5.6. Determination of the Total Phenolic Content and Antioxidant Capacity
2.5.7. Microscope Observation
2.5.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Mango Peel Extract
3.2. Evaluation of the Solid Shampoo Formulations
3.2.1. Determination of the Antioxidant Potential
3.2.2. Surface Tension and pH Value
3.2.3. Dirt Dispersion Test
3.2.4. Accelerated Thermal Stability Test
3.2.5. Oxidative Stability
3.2.6. Microscope Observation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kılıç, Z. The importance of water and conscious use of water. Int. J. Hydrol. 2020, 4, 239–241. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Y.; Xia, J. Hydrological cycle and water resources in a changing world: A review. Geogr. Sustain. 2021, 2, 115–122. [Google Scholar] [CrossRef]
- Hussain, T.; Wahab, A. A critical review of the current water conservation practices in textile wet processing. J. Clean. Prod. 2018, 198, 806–819. [Google Scholar] [CrossRef]
- Programme, U.W.W.A. The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; United Nations: New York, NY, USA, 2023; p. 189. [Google Scholar]
- Gleick, P.H.; Cooley, H. Freshwater Scarcity. Annu. Rev. Environ. Resour. 2021, 46, 319–348. [Google Scholar] [CrossRef]
- Cosmetics Europe. Cosmetics and Personal Care Industry Overview. Available online: https://cosmeticseurope.eu/cosmetics-industry/ (accessed on 23 July 2023).
- Aguiar, J.B.; Martins, A.M.; Almeida, C.; Ribeiro, H.M.; Marto, J. Water sustainability: A waterless life cycle for cosmetic products. Sustain. Prod. Consum. 2022, 32, 35–51. [Google Scholar] [CrossRef]
- Gatt, I.J.; Refalo, P. Reusability and recyclability of plastic cosmetic packaging: A life cycle assessment. Resour. Conserv. Recycl. Adv. 2022, 15, 200098. [Google Scholar] [CrossRef]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro)plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Cinelli, P.; Coltelli, M.B.; Signori, F.; Morganti, P.; Lazzeri, A. Cosmetic Packaging to Save the Environment: Future Perspectives. Cosmetics 2019, 6, 26. [Google Scholar] [CrossRef]
- Sahota, A. Sustainability: How the Cosmetics Industry Is Greening up; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A step forward on sustainability in the cosmetics industry: A review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef]
- Soto, M.L.; Falqué, E.; Domínguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Manhongo, T.T.; Chimphango, A.; Thornley, P.; Röder, M. Techno-economic and environmental evaluation of integrated mango waste biorefineries. J. Clean. Prod. 2021, 325, 129335. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Gokhale, S.; Rathod, V.K. Utilization of waste mango peels for extraction of polyphenolic antioxidants by ultrasound-assisted natural deep eutectic solvent. Bioresour. Technol. Rep. 2022, 18, 101074. [Google Scholar] [CrossRef]
- Bai, X.; Lai, T.; Zhou, T.; Li, Y.; Li, X.; Zhang, H. In vitro antioxidant activities of phenols and oleanolic acid from mango peel and their cytotoxic effect on A549 cell line. Molecules 2018, 23, 1395. [Google Scholar] [CrossRef]
- Páramos, P.R.S.; Granjo, J.F.O.; Corazza, M.L.; Matos, H.A. Extraction of high value products from avocado waste biomass. J. Supercrit. Fluids 2020, 165, 104988. [Google Scholar] [CrossRef]
- Fernandes, F.; Gorissen, K.; Delerue-Matos, C.; Grosso, C. Valorisation of Agro-Food By-Products for the Extraction of Phenolic Compounds. Biol. Life Sci. Forum 2022, 18, 61. [Google Scholar] [CrossRef]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef]
- Seifu, T.; Abera, A. Extraction of essential oil from orange peel using different methods and effect of solvents, time, temperature to maximize yield. Int. J. Eng. Sci. Comput. 2019, 9, 24300–24308. [Google Scholar]
- Bourgou, S.; Rahali, F.Z.; Ourghemmi, I.; Saïdani Tounsi, M. Changes of Peel Essential Oil Composition of Four Tunisian Citrus during Fruit Maturation. Sci. World J. 2012, 2012, 528593. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Deng, W.; Hu, W.; Cao, S.; Zhong, B.; Chun, J. Extraction of ‘Gannanzao’orange peel essential oil by response surface methodology and its effect on cancer cell proliferation and migration. Molecules 2019, 24, 499. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Nouli, E.; Xekoukoulotakis, N.P.; Mantzavinos, D. Effect of key operating parameters on phenols degradation during H2O2-assisted TiO2 photocatalytic treatment of simulated and actual olive mill wastewaters. Appl. Catal. B Environ. 2007, 73, 11–22. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. From by-product to functional ingredient: Incorporation of avocado peel extract as an antioxidant and antibacterial agent. Innov. Food Sci. Emerg. Technol. 2022, 80, 103116. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Umbach, W. Cosmetics and Toiletries: Development, Production and Use; Ellis Horwood: New York, NY, USA, 1991. [Google Scholar]
- Al Badi, K.; Khan, S.A. Formulation, evaluation and comparison of the herbal shampoo with the commercial shampoos. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 301–305. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- del Pilar Sánchez-Camargo, A.; Ballesteros-Vivas, D.; Buelvas-Puello, L.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Cifuentes, A.; Ferreira, S.R.; Gutiérrez, L.-F. Microwave-assisted extraction of phenolic compounds with antioxidant and anti-proliferative activities from supercritical CO2 pre-extracted mango peel as valorization strategy. LWT 2021, 137, 110414. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Ballesteros Vivas, D.; Ortega Barbosa, J.; Morantes Medina, S.J.; Aristizabal Gutiérrez, F.; Parada-Alfonso, F. Bioactive phenolic compounds from the agroindustrial waste of Colombian mango cultivars ‘Sugar Mango’and ‘Tommy Atkins’—An alternative for their use and valorization. Antioxidants 2019, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Fitrasyah, S.I.; Ariani, A.; Rahman, N.; Nurulfuadi, N.; Aiman, U.; Nadila, D.; Pradana, F.; Rakhman, A.; Hartini, D.A. Analysis of Chemical Properties and Antioxidant Activity of Sambiloto (Andrographis paniculata Nees.) Leaf Tea Formula as a Functional Drink in Preventing Coronavirus Diseases and Degenerative Diseases. Open Access Maced. J. Med. Sci. (OAMJMS) 2021, 9, 196–201. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Gomes, S.M.; Santos, L. A Novel Approach in Skin Care: By-Product Extracts as Natural UV Filters and an Alternative to Synthetic Ones. Molecules 2023, 28, 2037. [Google Scholar] [CrossRef]
- Markom, M.; Hasan, M.; Daud, W.R.W.; Singh, H.; Jahim, J.M. Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: Effects of solvents and extraction methods. Sep. Purif. Technol. 2007, 52, 487–496. [Google Scholar] [CrossRef]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of bioactive compounds from mango (Mangifera indica L. var. Carabao) seed kernel with ethanol–water binary solvent systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- AlQuadeib, B.T.; Eltahir, E.K.D.; Banafa, R.A.; Al-Hadhairi, L.A. Pharmaceutical evaluation of different shampoo brands in local Saudi market. Saudi Pharm. J. 2018, 26, 98–106. [Google Scholar] [CrossRef]
- Dias, M.F.R.G.; de Almeida, A.M.; Cecato, P.M.R.; Adriano, A.R.; Pichler, J. The shampoo pH can affect the hair: Myth or reality? Int. J. Trichol. 2014, 6, 95. [Google Scholar] [CrossRef]
- Maeda, K.; Yamazaki, J.; Okita, N.; Shimotori, M.; Igarashi, K.; Sano, T. Mechanism of Cuticle Hole Development in Human Hair Due to UV-Radiation Exposure. Cosmetics 2018, 5, 24. [Google Scholar] [CrossRef]







| Phase | Ingredient | Function | % (w/w) | |||
|---|---|---|---|---|---|---|
| A | Sodium Cocoyl Isethionate | Primary Surfactant | 65.5 | |||
| Coco Glucoside | Secondary Surfactant | 20 | ||||
| B | Beeswax | Consistency adjustment | 8 | |||
| Shea Butter | Emollient | 5 | ||||
| Panthenol | Humectant | 1 | ||||
| D | Essential Oil | Fragrance | 0.5 | |||
| E | Lactic Acid | Buffer | 0.05 | |||
| Formulation | ||||||
| Additives (Phase C) | NC | PC1 | PC2 | M1 | M2 | Mix |
| Tocopherol | - | 1 | - | - | - | 0.5 |
| BHT | - | - | 0.5 | - | - | - |
| MP Extract | - | - | - | 1 | 2 | 0.5 |
| TPC (mgGAE·g−1extract) | 12.4 ± 0.8 | ||
| DPPH | IC50 (mgextract·L−1) | 51.5 ± 2.0 | |
| TE/ mgTrolox·g−1extract | 330.5 ± 13.1 | ||
| ABTS | IC50 (mgextract·L−1) | 28.6 ± 1.0 | |
| TEAC (mgTrolox·g−1extract) | 315.0 ± 18.1 | ||
| HPLC-DAD | |||
| Compound Identified | Concentration (µg·gdried extract−1) | ||
| Catechin | 192.0 ± 3.2 | ||
| Chlorogenic Acid | 46.8 ± 3.6 | ||
| Quercetin | 114.0 ± 1.9 | ||
| Rosmarinic Acid | 186.1 ± 1.1 | ||
| Extraction Method | TPC/mgGAE∙g−1 | AO Capacity/mg∙L−1 | Main Phenolic Compounds | Reference | |
|---|---|---|---|---|---|
| Soxhlet Ethanol | 55.8 | IC50 =51.1 | Gallic acid Chlorogenic acid | Catechin Kaempferol | [19] |
| MAE Ethanol:Water (60:40) | 52.1 | IC50 =23.0 | Gallic acid Mangiferin | Quercetin Quinic acid | [34] |
| MAE Ethanol:Water (70:30) | 0.7 | - | Oleanolic acid Chlorogenic acid | Gallic acid Caffeic acid | [20] |
| Soxhlet Methanol | 18.4 | - | Gallic acid derivatives | Mangiferin | [35] |
| Maceration Ethanol:Water (70:30) | 27.5 | - | Gallic acid Chlorogenic acid | Catechin Quercetin | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, I.; Ferreira, S.M.; Santos, L. On the Path to Sustainable Cosmetics: Development of a Value-Added Formulation of Solid Shampoo Incorporating Mango Peel Extract. Cosmetics 2023, 10, 140. https://doi.org/10.3390/cosmetics10050140
Brito I, Ferreira SM, Santos L. On the Path to Sustainable Cosmetics: Development of a Value-Added Formulation of Solid Shampoo Incorporating Mango Peel Extract. Cosmetics. 2023; 10(5):140. https://doi.org/10.3390/cosmetics10050140
Chicago/Turabian StyleBrito, Inês, Sara M. Ferreira, and Lúcia Santos. 2023. "On the Path to Sustainable Cosmetics: Development of a Value-Added Formulation of Solid Shampoo Incorporating Mango Peel Extract" Cosmetics 10, no. 5: 140. https://doi.org/10.3390/cosmetics10050140
APA StyleBrito, I., Ferreira, S. M., & Santos, L. (2023). On the Path to Sustainable Cosmetics: Development of a Value-Added Formulation of Solid Shampoo Incorporating Mango Peel Extract. Cosmetics, 10(5), 140. https://doi.org/10.3390/cosmetics10050140