Adaptogen Technology for Skin Resilience Benefits
Abstract
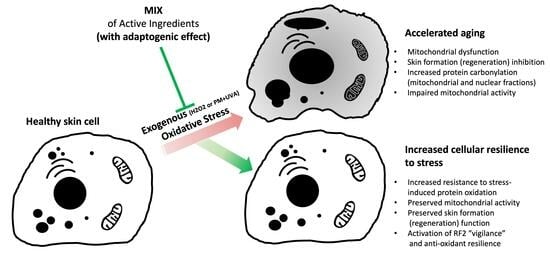
:1. Introduction
- -
- An extract of Withania somnifera roots (or Ashwagandha), an adaptogen plant with a long history of use in Ayurvedic medicine for promoting longevity and slowing aging;
- -
- A ferment of Lactobacillus plantarum, known for its beneficial effects on the skin’s barrier function;
- -
- A superfruit extract of the Kakadu plum (Terminalia ferdinandiana), which is the richest natural source of vitamin C.
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Expression Proteomics Analysis and Ingenuity Pathway Analysis (IPA)
2.3. Mitochondrial Protein and Nuclear Protein (Histone-Enriched) Fraction Preparation
2.4. Protein Carbonylation Analysed
2.5. Mitochondrial Function Analysis
3. Results and Discussion
3.1. MS (Mass Spectrometry)-Based Proteomic Analysis
3.2. Carbonylation of Proteins
3.3. Mitochondrial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care is Safer Care; World Health Organization—WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Fussell, J.C.; Kelly, F.J. Oxidative contribution of air pollution to extrinsic skin ageing. Free Radic. Biol. Med. 2020, 151, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the ski’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar]
- Birch-Machin, M.A.; Russell, E.V.; Latimer, J.A. Mitochondrial DNA damage as a biomarker for ultraviolet radiation exposure and oxidative stress. Br. J. Dermatol. 2013, 169 (Suppl. 2), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.; Bowman, A.; Rashdan, E.; Birch-Machin, M.A. Mitochondrial damage and ageing using skin as a model organ. Maturitas 2016, 93, 34–40. [Google Scholar] [CrossRef]
- Dezest, M.; Le Bechec, M.; Chavatte, L.; Desauziers, V.; Chaput, B.; Grolleau, J.L.; Descargues, P.; Nizard, C.; Schnebert, S.; Lacombe, S.; et al. Oxidative damage and impairment of protein quality control systems in keratinocytes exposed to a volatile organic compounds cocktail. Sci. Rep. 2017, 7, 10707. [Google Scholar] [CrossRef]
- Cavagnino, A.; Bobier, A.; Baraibar, M. The skin Oxi-Proteome as a molecular signature of exposome stress. H&PC Today 2019, 14, 4. [Google Scholar]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int J Mol Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef]
- Nyström, T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005, 24, 1311–1317. [Google Scholar] [CrossRef]
- Levine, R.L. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic. Biol. Med. 2002, 32, 790–796. [Google Scholar] [CrossRef]
- Zhang, H. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88 Pt B, 314–336. [Google Scholar] [CrossRef]
- Lavigne, E.G.; Cavagnino, A.; Steinschneider, R.; Breton, L.; Baraibar, M.A.; Jäger, S. Oxidative damage prevention in human skin and sensory neurons by a salicylic acid derivative. Free Radic. Biol. Med. 2022, 181, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C. What has passed is prolog: New cellular and physiological roles of G6PD. Free Radic. Res. 2016, 50, 1047–1064. [Google Scholar] [CrossRef]
- Nobrega-Pereira, S. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef] [PubMed]
- Frohnert, B.I.; Bernlohr, D.A. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Adv. Nutr. 2013, 4, 157–163. [Google Scholar] [CrossRef] [PubMed]
- McCormack, J.G.; Halestrap, A.P.; Denton, R.M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990, 70, 391–425. [Google Scholar] [CrossRef]
- He, W.; Newman, J.C.; Wang, M.Z.; Ho, L.; Verdin, E. Mitochondrial sirtuins: Regulators of protein acylation and metabolism. Trends Endocrinol. Metab. 2012, 23, 467–476. [Google Scholar] [CrossRef]
- Tzameli, I. The evolving role of mitochondria in metabolism. Trends Endocrinol. Metab. 2012, 23, 417–419. [Google Scholar] [CrossRef]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Noguchi, M.; Kasahara, A. Mitochondrial dynamics coordinate cell differentiation. Biochem. Biophys. Res. Commun. 2018, 500, 59–64. [Google Scholar] [CrossRef]
- Chandel, N.S. Mitochondria as signaling organelles. BMC Biol. 2014, 12, 34. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem. Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Passos, J.F. Reactive Oxygen Species Detection in Senescent Cells. Methods Mol. Biol. 2019, 1896, 21–29. [Google Scholar]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schroeder, P. Role of mitochondria in photoaging of human skin: The defective powerhouse model. J. Investig. Dermatol. Symp. Proc. 2009, 14, 44–49. [Google Scholar] [CrossRef]
- Naidoo, K.; Hanna, R.; Birch-Machin, M.A. What is the role of mitochondrial dysfunction in skin photoaging? Exp. Dermatol. 2018, 27, 124–128. [Google Scholar] [CrossRef]
- Cabrera, F.; Ortega, M.; Velarde, F.; Parra, E.; Gallardo, S.; Barba, D.; Soto, L.; Peña, G.; Pedroza, L.A.; Jorgensen, C.; et al. Primary allogeneic mitochondrial mix (PAMM) transfer/transplant by MitoCeption to address damage in PBMCs caused by ultraviolet radiation. BMC Biotechnol. 2019, 19, 42. [Google Scholar] [CrossRef]
- Moller, I.M. Plant mitochondria and oxidative stress: Electron Transport, NADPH Turnover, and Metabolism of Reactive Oxygen Species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Laborit, H. Éloge de la Fuite; Robert Laffont: Paris, France, 1976. [Google Scholar]
- Simonet, G. Le concept d’adaptation: Polysémie interdisciplinaire et implication pour les changements climatiques. Nat. Sci. Sociétés 2009, 17, 392–401. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34 (Suppl. 4), 4–25. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.A.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Wagner, H. Plant adaptogens III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine 1999, 6, 287–300. [Google Scholar] [CrossRef]
- Panossian, A.; Gabrielian, E.; Wagner, H. On the mechanism of action of plant adaptogens with particular reference to cucurbitacin R diglucoside. Phytomedicine 1999, 6, 147–155. [Google Scholar] [CrossRef]
- Panossian, A. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.J.; Efferth, T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 2018, 50, 257–284. [Google Scholar] [CrossRef]
- Lazarev, N.V. General and specific in action of pharmacological agents. Farmacol. Toxicol. 1958, 21, 81–86. [Google Scholar]
- Lazarev, N.V.; Ljublina, E.I.; Ljublina, M.A. State of nonspecific resistance. Patol. Fiziol. Exp. Terapia. 1959, 3, 16–21. [Google Scholar]
- Yadav, B.; Bajaj, A.; Saxena, M.; Saxena, A.K. In Vitro Anticancer Activity of the Root, Stem and Leaves of Withania Somnifera against Various Human Cancer Cell Lines. Indian J. Pharm. Sci. 2010, 72, 659–663. [Google Scholar] [CrossRef]
- Ilayperuma, I.; Ratnasooriya, W.D.; Weerasooriya, T.R. Effect of Withania somnifera root extract on the sexual behaviour of male rats. Asian J. Androl. 2002, 4, 295–298. [Google Scholar] [PubMed]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Altaf, M.; Bukhari, S.N.A. Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran. J. Basic Med. Sci. 2020, 23, 1501–1526. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Baghirova, S.; Hughes, B.G.; Hendzel, M.J.; Schulz, R. Sequential fractionation and isolation of subcellular proteins from tissue or cultured cells. MethodsX 2015, 2, 440–445. [Google Scholar] [CrossRef]
- Baraibar, M.A.; Ladouce, R.; Friguet, B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. J. Proteom. 2013, 92, 63–70. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kawamata, H.; Tiranti, V.; Magrané, J.; Chinopoulos, C.; Manfredi, G. adPEO mutations in ANT1 impair ADP-ATP translocation in muscle mitochondria. Hum. Mol. Genet. 2011, 20, 2964–2974. [Google Scholar] [CrossRef]
- Brand, M.D.; Pakay, J.L.; Ocloo, A.; Kokoszka, J.; Wallace, D.C.; Brookes, P.S.; Cornwall, E.J. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 2005, 392 Pt 2, 353–362. [Google Scholar] [CrossRef]
- Kim, E.H.; Koh, E.H.; Park, J.Y.; Lee, K.U. Adenine nucleotide translocator as a regulator of mitochondrial function: Implication in the pathogenesis of metabolic syndrome. Korean Diabetes J. 2010, 34, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Folco, E.G.; Lee, C.S.; Dufu, K.; Yamazaki, T.; Reed, R. The proteins PDIP3 and ZC11A associate with the human TREX complex in an ATP-dependent manner and function in mRNA export. PLoS ONE 2012, 7, e43804. [Google Scholar] [CrossRef] [PubMed]
- Sumara, I.; Vorlaufer, E.; Gieffers, C.; Peters, B.H.; Peters, J.M. Characterization of vertebrate cohesin complexes and their regulation in prophase. J. Cell Biol. 2000, 151, 749–762. [Google Scholar] [CrossRef]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Matsumaru, D.; Motohashi, H. The KEAP1-NRF2 System in Healthy Aging and Longevity. Antioxidants 2021, 10, 1929. [Google Scholar] [CrossRef]
- Chayen, J.; Howat, D.W.; Bitensky, L. Cellular biochemistry of glucose 6-phosphate and 6-phosphogluconate dehydrogenase activities. Cell Biochem. Funct. 1986, 4, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369. [Google Scholar] [CrossRef]
- White, K.; Someya, S. The roles of NADPH and isocitrate dehydrogenase in cochlear mitochondrial antioxidant defense and aging. Hear. Res. 2023, 427, 108659. [Google Scholar] [CrossRef]
- Kochetov, G.A.; Solovjeva, O.N. Structure and functioning mechanism of transketolase. Biochim. Biophys. Acta 2014, 1844, 1608–1618. [Google Scholar] [CrossRef]
- Moriyama, T.; Tanaka, S.; Nakayama, Y.; Fukumoto, M.; Tsujimura, K.; Yamada, K.; Bamba, T.; Yoneda, Y.; Fukusaki, E.; Oka, M. Two isoforms of TALDO1 generated by alternative translational initiation show differential nucleocytoplasmic distribution to regulate the global metabolic network. Sci. Rep. 2016, 6, 34648. [Google Scholar] [CrossRef]
- Xu, M.; Ding, L.; Liang, J.; Yang, X.; Liu, Y.; Wang, Y.; Ding, M.; Huang, X. NAD kinase sustains lipogenesis and mitochondrial metabolismthrough fatty acid synthesis. Cell Rep. 2021, 37, 110157. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.; Jain, A.P.; Nanjappa, V.; Patel, K.; Mangalaparthi, K.K.; Babu, N.; Cavusoglu, N.; Roy, N.; Soeur, J.; Breton, L.; et al. Proteome-wide changes in primary skin keratinocytes exposed to diesel particulate extract-A role for antioxidants in skin health. J. Dermatol. Sci. 2019, 96, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Lourenço Dos Santos, S.; Baraibar, M.A.; Lundberg, S.; Eeg-Olofsson, O.; Larsson, L.; Friguet, B. Oxidative proteome alterations during skeletal muscle ageing. Redox Biol. 2015, 5, 267–274. [Google Scholar] [CrossRef] [PubMed]




| Accession Number | Description | MIX (in Basal Conditions) | Stress (H2O2) | MIX + Stress | |||
|---|---|---|---|---|---|---|---|
| Fold Change (vs. Ctrl) | p-Value | Fold Change (vs. Ctrl) | p-Value | Fold Change (vs. Stress) | p-Value | ||
| P12235 | ADP/ATP translocase 1 | +1.14 | 0.525 | −2.09 | 0.039 | +2.37 | 0.007 |
| Q9BY77 | Polymerase delta-interacting protein 3 | −1.08 | 0.780 | −2.63 | 0.007 | +2.43 | 0.012 |
| Q9UQE7 | Structural maintenance of chromosome protein 3 | −1.87 | 0.416 | −6.13 | 0.006 | +5.53 | 0.020 |
| Analysis | MIX (in Basal Condition) (vs. Ctrl) | Stress (H2O2) (vs. Ctrl) | MIX + Stress (vs. Ctrl) | |||
|---|---|---|---|---|---|---|
| z-Score | p-Value | z-Score | p-Value | z-Score | p-Value | |
| Canonical Pathway Oxidative Phosphorylation | 2.309 | <0.001 | NaN | 0.48 | 2.449 | 0.035 |
| Upstream Analysis Nuclear factor, erythroid 2 like 2 (NRF2) | NaN | <0.001 | NaN | / | 2.236 | <0.001 |
| Biofunction Formation (Regeneration) of the skin | −1.091 | <0.001 | −1.715 | <0.001 | NaN | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavagnino, A.; Breton, L.; Ruaux, C.; Grossgold, C.; Levoy, S.; Abdayem, R.; Roumiguiere, R.; Cheilian, S.; Bouchara, A.; Baraibar, M.A.; et al. Adaptogen Technology for Skin Resilience Benefits. Cosmetics 2023, 10, 155. https://doi.org/10.3390/cosmetics10060155
Cavagnino A, Breton L, Ruaux C, Grossgold C, Levoy S, Abdayem R, Roumiguiere R, Cheilian S, Bouchara A, Baraibar MA, et al. Adaptogen Technology for Skin Resilience Benefits. Cosmetics. 2023; 10(6):155. https://doi.org/10.3390/cosmetics10060155
Chicago/Turabian StyleCavagnino, Andrea, Lionel Breton, Charline Ruaux, Celeste Grossgold, Suzy Levoy, Rawad Abdayem, Romain Roumiguiere, Stephanie Cheilian, Anne Bouchara, Martin A. Baraibar, and et al. 2023. "Adaptogen Technology for Skin Resilience Benefits" Cosmetics 10, no. 6: 155. https://doi.org/10.3390/cosmetics10060155
APA StyleCavagnino, A., Breton, L., Ruaux, C., Grossgold, C., Levoy, S., Abdayem, R., Roumiguiere, R., Cheilian, S., Bouchara, A., Baraibar, M. A., & Gueniche, A. (2023). Adaptogen Technology for Skin Resilience Benefits. Cosmetics, 10(6), 155. https://doi.org/10.3390/cosmetics10060155