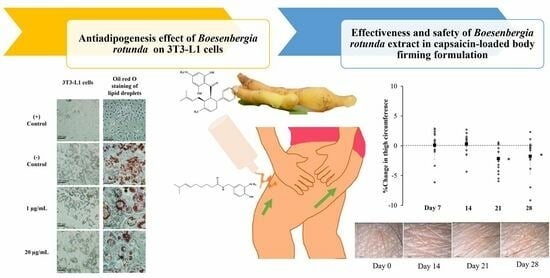
Effectiveness and Safety of Boesenbergia rotunda Extract on 3T3-L1 Preadipocytes and Its Use in Capsaicin-Loaded Body-Firming Formulation: In Vitro Biological Study and In Vivo Human Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cytotoxicity Study of B. rotunda on 3T3-L1 Cells
2.3. Antiadipogenesis Evaluation with Oil Red O Staining Assay
2.4. Preparation of B. rotunda in Body-Firming Formulation
2.5. HPLC Analysis
2.6. In Vitro Skin Permeation and Deposition Study
2.7. In Vivo Human Skin Study
2.8. Data Analysis
3. Results
3.1. Bioactivity of B. rotunda Extracts on 3T3-L1 Cells
3.2. Physicochemical Properties of B. rotunda in Capsaicin Body-Firming Formulations
3.3. In Vivo Human Skin Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escalante, G.; Bryan, P.; Rodriguez, J. Effects of a topical lotion containing aminophylline, caffeine, yohimbe, l-carnitine, and gotu kola on thigh circumference, skinfold thickness, and fat mass in sedentary females. J. Cosmet. Dermatol. 2019, 18, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Mittal, B. Subcutaneous adipose tissue & visceral adipose tissue. Indian J. Med. Res. 2019, 149, 571–573. [Google Scholar] [PubMed]
- Dixit, V.V.; Wagh, M.S. Unfavourable outcomes of liposuction and their management. Indian J. Plast. Surg. 2013, 46, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Snitker, S.; Fujishima, Y.; Shen, H.; Ott, S.; Pi-Sunyer, X.; Furuhata, Y.; Ohinata, K. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: Possible pharmacogenetic implications. Am. J. Clin. Nutr. 2009, 89, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Kawai, Y.; Iwanaga, T.; Saito, M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr. 2012, 95, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Tate, S.; Woolf, C.J.; Caterina, M.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef]
- Kim, M.S.; Pyun, H.B.; Hwang, J.K. Effects of orally administered fingerroot (Boesenbergia pandurata) extract on oxazolone-induced atopic dermatitis-like skin lesions in hairless mice. Food Sci. Biotechnol. 2013, 22, 257–264. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.S.; Sa, B.K.; Kim, M.B.; Hwang, J.K. Boesenbergia pandurata attenuates diet-induced obesity by activating AMP-activated protein kinase and regulating lipid metabolism. Int. J. Mol. Sci. 2012, 13, 994–1005. [Google Scholar] [CrossRef]
- Jitsaeng, K.; Duangjit, S.; Sritananuwat, P.; Tansathien, K.; Opanasopit, P.; Rangsimawong, W. Potential Lipolytic Effect of Panduratin A Loaded Microspicule Serum as a Transdermal Delivery Approach for Subcutaneous Fat Reduction. Biol. Pharm. Bull. 2023, 46, 1770–1777. [Google Scholar] [CrossRef]
- Nobusue, H.; Endo, T.; Kano, K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue. Cell Tissue Res. 2008, 332, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Rangsimawong, W.; Jitsaeng, K.; Duangjit, S.; Sritananuwat, P.; Opanasopit, P. Development of isolated panduratin A-loaded solid lipid nanoparticles as a transdermal delivery system for cosmeceutical products. Sci. Eng. Health Stud. 2023, 17, 23050003. [Google Scholar]
- Tan, B.C.; Tan, S.K.; Wong, S.M.; Ata, N.; Rahman, N.A.; Khalid, N. Distribution of Flavonoids and Cyclohexenyl Chalcone Derivatives in Conventional Propagated and In Vitro-Derived Field-Grown Boesenbergia rotunda (L.) Mansf. Evid. Based Complement. Altern. Med. 2015, 2015, 451870. [Google Scholar] [CrossRef]
- Elrayess, M.A.; Almuraikhy, S.; Kafienah, W.; Al-Menhali, A.; Al-Khelaifi, F.; Bashah, M.; Jaganjac, M. 4-hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance. Free Radic. Biol. Med. 2017, 104, 129–137. [Google Scholar] [CrossRef] [PubMed]
- San, H.T.; Khine, H.E.E.; Sritularak, B.; Prompetchara, E.; Chaotham, C.; Che, C.T.; Likhitwitayawuid, K. Pinostrobin: An Adipogenic Suppressor from Fingerroot (Boesenbergia rotunda) and Its Possible Mechanisms. Foods 2022, 11, 3024. [Google Scholar] [CrossRef]
- Kim, D.; Lee, M.S.; Jo, K.; Lee, K.E.; Hwang, J.K. Therapeutic potential of panduratin A, LKB1-dependent AMP-activated protein kinase stimulator, with activation of PPARalpha/delta for the treatment of obesity. Diabetes Obes. Metab. 2011, 13, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Chumchoochart, W.; Sutthanut, K. Anti-obesity potential of glutinous black rice bran extract: Anti-adipogenesis and lipolysis induction in 3T3-L1 adipocyte model. Songklanakarin J. Sci. Technol. 2020, 42, 284–291. [Google Scholar]
- Thummajitsakul, S.; Silprasit, K. Classification of some Boesenbergia and Alpinia extracts and their medicinal products based on chemical composition, antioxidant activity, and concentration of some heavy metals. Songklanakarin J. Sci. Technol. 2021, 43, 160–168. [Google Scholar]
- Kosuwon, W.; Sirichatiwapee, W.; Wisanuyotin, T.; Jeeravipoolvarn, P.; Laupattarakasem, W. Efficacy of symptomatic control of knee osteoarthritis with 0.0125% of capsaicin versus placebo. J. Med. Assoc. Thai 2010, 93, 1188–1195. [Google Scholar] [PubMed]
- Tan, E.-C.; Lee, Y.-K.; Chee, C.-F.; Heh, C.-H.; Wong, S.-M.; Christina Thio, L.P.; Foo, G.-T.; Khalid, N.; Abd Rahman, N.; Karsani, S.A.; et al. Boesenbergia rotunda: From Ethnomedicine to Drug Discovery. Evid. Based Complement. Altern. Med. 2012, 2012, 473637. [Google Scholar]
- Zhang, L.L.; Yan Liu, D.; Ma, L.Q.; Luo, Z.D.; Cao, T.B.; Zhong, J.; Zhu, M.X. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res. 2007, 100, 1063–1070. [Google Scholar] [CrossRef]
- Altman, R.; Barkin, R.L. Topical therapy for osteoarthritis: Clinical and pharmacologic perspectives. Postgrad. Med. 2009, 121, 139–147. [Google Scholar] [CrossRef]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef]
- Rao, J.; Paabo, K.E.; Goldman, M.P. A double-blinded randomized trial testing the tolerability and efficacy of a novel topical agent with and without occlusion for the treatment of cellulite: A study and review of the literature. J. Drugs Dermatol. 2004, 3, 417–425. [Google Scholar]
- Nanok, K.; Sansenya, S. α-Glucosidase, α-amylase, and tyrosinase inhibitory potential of capsaicin and dihydrocapsaicin. J. Food Biochem. 2020, 44, e13099. [Google Scholar] [CrossRef]
- Yoon, J.H.; Shim, J.S.; Cho, Y.; Baek, N.I.; Lee, C.W.; Kim, H.S.; Hwang, J.K. Depigmentation of melanocytes by isopanduratin A and 4-hydroxypanduratin A isolated from Kaempferia pandurata ROXB. Biol. Pharm. Bull. 2007, 30, 2141–2145. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Kim, H.S.; Kim, H.K.; Kim, J.W.; Yoon, J.H.; Cho, Y.; Hwang, J.K. Inhibitory effect of panduratin A isolated from Kaempferia panduarata Roxb. on melanin biosynthesis. Phytother. Res. 2010, 24, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Rice, A.S.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 1, CD007393. [Google Scholar] [PubMed]
- Chrubasik, S.; Weiser, T.; Beime, B. Effectiveness and safety of topical capsaicin cream in the treatment of chronic soft tissue pain. Phytother. Res. 2010, 24, 1877–1885. [Google Scholar] [CrossRef]
- Serup, J.; Jemec, G.B.E.; Grove, G. Handbook of Non-Invasive Methods and the Skin, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Oliveira, R.; Ferreira, J.; Azevedo, L.F.; Almeida, I.F. An Overview of Methods to Characterize Skin Type: Focus on Visual Rating Scales and Self-Report Instruments. Cosmetics 2023, 10, 14. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.W.; Rhim, D.B.; Kim, C.; Hwang, J.K. Effect of Standardized Boesenbergia pandurata Extract and Its Active Compound Panduratin A on Skin Hydration and Barrier Function in Human Epidermal Keratinocytes. Prev. Nutr. Food Sci. 2015, 20, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; Thi, T.T.; Nguyen, H.T.; Lao, T.D.; Binh, N.T.; Nguyen, Q.D. Anti-Aging Effects of a Serum Based on Coconut Oil Combined with Deer Antler Stem Cell Extract on a Mouse Model of Skin Aging. Cells 2022, 11, 597. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sritananuwat, P.; Samseethong, T.; Jitsaeng, K.; Duangjit, S.; Opanasopit, P.; Rangsimawong, W. Effectiveness and Safety of Boesenbergia rotunda Extract on 3T3-L1 Preadipocytes and Its Use in Capsaicin-Loaded Body-Firming Formulation: In Vitro Biological Study and In Vivo Human Study. Cosmetics 2024, 11, 24. https://doi.org/10.3390/cosmetics11010024
Sritananuwat P, Samseethong T, Jitsaeng K, Duangjit S, Opanasopit P, Rangsimawong W. Effectiveness and Safety of Boesenbergia rotunda Extract on 3T3-L1 Preadipocytes and Its Use in Capsaicin-Loaded Body-Firming Formulation: In Vitro Biological Study and In Vivo Human Study. Cosmetics. 2024; 11(1):24. https://doi.org/10.3390/cosmetics11010024
Chicago/Turabian StyleSritananuwat, Phaijit, Tipada Samseethong, Kusuma Jitsaeng, Sureewan Duangjit, Praneet Opanasopit, and Worranan Rangsimawong. 2024. "Effectiveness and Safety of Boesenbergia rotunda Extract on 3T3-L1 Preadipocytes and Its Use in Capsaicin-Loaded Body-Firming Formulation: In Vitro Biological Study and In Vivo Human Study" Cosmetics 11, no. 1: 24. https://doi.org/10.3390/cosmetics11010024
APA StyleSritananuwat, P., Samseethong, T., Jitsaeng, K., Duangjit, S., Opanasopit, P., & Rangsimawong, W. (2024). Effectiveness and Safety of Boesenbergia rotunda Extract on 3T3-L1 Preadipocytes and Its Use in Capsaicin-Loaded Body-Firming Formulation: In Vitro Biological Study and In Vivo Human Study. Cosmetics, 11(1), 24. https://doi.org/10.3390/cosmetics11010024