High-Resolution Mass Spectrometry for the Comprehensive Characterization of Plant-Pigment-Based Tattoos and Dyes Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Samples
2.3. Sample Preparation
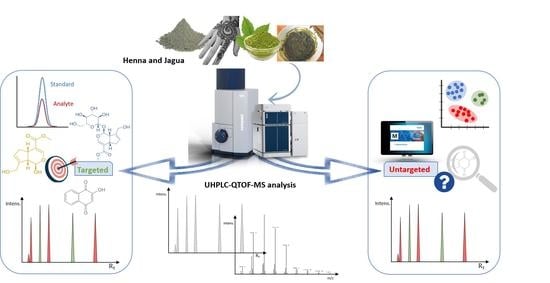
2.4. Ultra-High-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (UHPLC-QOF-MS)
3. Results and Discussion
3.1. Chromatographic Analysis
3.2. Application to Real Samples
3.3. Identification of Untargeted Analytes by UHPLC-QTOF
4. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kazandjieva, J.; Grozdev, L.; Tsankov, N. Temporary henna tattoos. Clin. Dermatol. 2007, 25, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Guerra, E.; Garcia-Jares, C.; Lores, M. Body-decorating products: Ingredients of permanent and temporary tattoos from analytical and european regulatory perspectives. Anal. Chim. Acta 2019, 1079, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.J.; Borrego, L.; Pulido-Melian, E.; Gonzalez-Diaz, O. Quantification of p-phenylenediamine and 2-hydroxy-1,4-naphthoquinone in henna tattoos. Contact Dermat. 2012, 66, 33–37. [Google Scholar] [CrossRef]
- Aktas Sukuroglu, A.; Battal, D.; Burgaz, S. Monitoring of lawsone, p-phenylenediamine and heavy metals in commercial temporary black henna tattoos sold in Turkey. Contact Dermat. 2017, 76, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Consumer Safety (SCCS) Opinion on Lawsonia Inermis (Henna) COLIPA n_ C169, 19 September 2013 SCCS/1511/13. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_140.pdf (accessed on 20 May 2021).
- Waton, J.; Brault, F.; Laveine, E. A putative case of allergic contact dermatitis caused by a jagua tattoo. Contact Dermat. 2017, 76, 296–321. [Google Scholar] [CrossRef]
- Maarouf, M.; Saberian, C.; Segal, R.J.; Shi, V.Y. A new era for tattoos, with new potential complications. J. Clin. Aesthet. Dermatol. 2019, 12, 37–38. [Google Scholar] [PubMed]
- Bircher, A.J.; Sigg, R.; Hofmeier, K.S.; Schlegel, U.; Hauri, U. Allergic contact dermatitis caused by a new temporary blue-black tattoo dye- sensitization to genipin from jagua (Genipa Americana L.) fruit extract. Contact Dermat. 2017, 77, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Manual of the Working Group on Cosmetic Products (Sub-Group on Borderline Products) on the Scope of Application of the Cosmetics Regulation (EC) No 1223/2009 (Art. 2(1)(A)) Version 3.1. 2017. Available online: https://ec.europa.eu/docsroom/documents/29002 (accessed on 20 May 2021).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast). Off. J. Eur. Union L 2009, 342, 59.
- Directive 2009/48/EC of the European Parliament and of the Council of 18 June 2009 on the Safety of Toys. Off. J. Eur. Union L 2009, 170, 1–37.
- Lores, M.; Celeiro, M.; Rubio, L.; Llompart, M.; Garcia-Jares, C. Extreme cosmetics and borderline products: An analytical-based survey of European regulation compliance. Anal. Bioanal. Chem. 2018, 410, 7085–7102. [Google Scholar] [CrossRef]
- Rubio, L.; Lores, M.; Garcia-Jares, C. Monitoring of natural pigments in henna and jagua tattoos for fake detection. Cosmetics 2020, 7, 74. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Ballesteros-Vivas, D.; Parada-Alfonso, F.; Ibañez, E.; Cifuentes, A. Recent applications of high resolution mass spectrometry for the characterization of plant natural products. Trends Anal. Chem. 2019, 112, 87–101. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Yan, G.; Wang, X. Recent developments and emerging trends of mass spectrometry for herbal ingredients analysis. Trends Anal. Chem. 2017, 94, 70–76. [Google Scholar] [CrossRef]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminform. 2016, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.; Schmidt, S.; Müller-Hannemann, M.; Neumann, S. In silico fragmentation for computer assisted identification of metabolite mass spectra. BMC Bioinform. 2010, 11, 148. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Santiago, M.; Priego-Capote, F.; Luque De Castro, M.D. Enhanced detection and identification in metabolomics by use of LC–MS/MS untargeted analysis in combination with gas-phase fractionation. Anal. Chem. 2014, 86, 7558–7565. [Google Scholar] [CrossRef]
- Ríos, J.J.; Roca, M.; Pérez-Gálvez, A. Systematic HPLC/ESI-high resolution-qTOF-MS methodology for metabolomic studies in nonfluorescent chlorophyll catabolites pathway. J. Anal. Methods Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Database. Available online: https://ec.europa.eu/growth/sectors/cosmetics/cosing_en (accessed on 20 May 2021).
- Scientific Committee on Consumer Safety Opinion (SCCS) on Fragrance Allergens in Cosmetic Products, 26–27 June 2012 SCCS/1459/11. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_102.pdf (accessed on 20 May 2021).
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Miniaturized matrix solid-phase dispersion followed by liquid chromatoraphy-tandem mass spectrometry for the quantification of synthetic dyes in cosmetics and foodstuffs used or consumed by children. J. Chromatogr. A 2017, 1529, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Nishioka, T. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 2, 523–526. [Google Scholar] [CrossRef]
- Folashade, K.O.; Omoregie, E.H. Chemical constituents and biological activity of medicinal plants used for the management of sickle cell disease—A review. J. Med. Plant Res. 2013, 7, 3452–3476. [Google Scholar]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Cartwright-Jones, C.; Viljoen, A. Lawsonia inermis L. (henna): Ethnobotanical, phytochemical and pharmacological aspects. J. Ethnopharmacol. 2014, 155, 80–103. [Google Scholar]
- Chaudhary, G.; Goyal, S.; Poonia, P. Lawsonia inermis Linnaeus: A phytopharmacological review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 91–98. [Google Scholar]
- Scientific Committee on Consumer Safety Opinion (SCCS) on Acid Orange 7, 18 June 2014, SCCS/1536/14, Revision of 23 September 2014. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_158.pdf (accessed on 20 May 2021).
- Labyad, N.; Aljele, S. Pharmacognostic and phytochemical study of hair dyes henna in Libyan market. IJPSAT 2020, 21, 239–244. [Google Scholar]
- Dweck, A.C. Natural ingredients for colouring and styling. Int. J. Cosmetic Sci. 2002, 24, 287–302. [Google Scholar] [CrossRef]


| Sample Code | Color/Aspect | Sample Type | Set | Labeled Ingredients | Additional Information Available in Label/Internet |
|---|---|---|---|---|---|
| HT-1 |  | Paste | 1 | None | 100% VEG, Indian origin, does not contain PPD, clinically tested. |
| HT-2 |  | ||||
| HT-3 |  | ||||
| HT-4 |  | ||||
| HT-5 |  | ||||
| HT-6 |  | ||||
| HT-7 |  | ||||
| HT-8 |  | ||||
| HT-9 |  | ||||
| HT-10 |  | Paste | 2 | None | 100% VEG, Indian origin. |
| HT-11 |  | ||||
| HT-12 |  | ||||
| HT-13 |  | Paste | 3 | None | Pakistani origin. |
| HT-14 |  | ||||
| HT-15 |  | Paste | 4 | None | 100% an ayuverdic and non-allergic medicated, mehndi with fruit and flower. Keep in cool, dry, and dark place. 100% VEG, Indian origin. |
| HT-16 |  | ||||
| HT-17 |  | ||||
| HTD-1 |  | Powder | - | Lawsonia inermis | 100% natural and pure, with 0% additives, 0% chemicals and no added dyes. Marrakech origin. Tips on how to use on hair. |
| HTD-2 |  | Powder | 5 | Fresh henna leaves | 100% pure and natural, no chemicals, preservatives, or pesticides, cruelty-free, parabens-free, vegan. Directions to use on hair, application, how to store. This product has been certified by the National Program for Organic Production, India Organic and USDA Organics. Best before: March 2022. |
| HTD-3 |  | Henna and Indigo | |||
| HD-1 |  | Powder | 6 | Henna plant powder (Lawsonia inermis), Juglans regia, Cassia obovata | Green product. Vegan. Free of ammonia, PPD and metal salts. |
| HD-2 |  | Indigofera tinctoria, Lawsonia inermis | |||
| HD-3 |  | Indigofera tinctoria, ferrous sulfate, Haematoxylon campechianum, Cassia obovata | |||
| HD-4 |  | Cassia obovata, Lawsonia inermis, sodium picramate | |||
| HD-5 |  | Lawsonia inermis | |||
| HD-6 |  | Cassia obovata, Curcuma longa, Chamomilla recutita | |||
| HD-7 |  | Powder | - | Lawsonia inermis (henna) leaf powder, Indian gooseberry (Amla), Soapnut (Ritha), Acacia concina (Shikakai), Bacopa monnieri (Brahmi), False daisy (Bhringraj) | 100% natural and pure. Cruelty-Free. No pesticides, no chemicals, and dyes. Does not contain ammonia, peroxides, PPD, heavy metals and other harmful compounds. For external use only, directions for use. Indian origin. |
| JT-1 |  | Paste | - | Genipa americana fruit juice, xanthan gum, citric acid, potassium sorbate, Lavandula angustifolia flower oil, limonene, linalool | Non tested on animals, non-toxic, PPD free, latex free. |
| JT-2 |  | Paste | - | Water, alcohol denat, Genipa americana, xanthan gum, citric acid, potassium sorbate | 100% Natural, for external use only. |
| JT-3 |  | Paste | - | Genipa americana fruit extract, xanthan gum, citric acid, Lavandula angustifolia herb o il, potassium sorbate | Dermatologically tested, vegan. |
| JT-4 |  | Powder | - | Genipa americana, sugar, xanthan gum, citric acid | Safe and non-toxic, not for use by children 12 years and under, adult supervision advised, non-permanent, 100% natural. |
| JT-5 |  | Paste | - | Water, glycerine, ethylhexylgycerol, genipa juice, hazelnut extract, pure green plant extract | Function, how to use and tips. Duration: 2 weeks. |
| JT-6 |  | Paste | - | Inorganic plant toner (28%), natural emulsifier (medical propylene glycol, 15%), gelatin water (8%), thickener (12%), medical distilled water (45%) | Magic tattoo pigment, color enhancer. Safe and natural, without PPD or other toxic chemicals. Duration: 2 weeks. |
| HPT |  | Liquid-paste | - | None | Herbaceous plant tattoo fluid. Vegetable dye, skin retention time varies between individuals because skin metabolism is slightly different. |
| Compound | Linearity | Mean Precision RSD (%) | LODs (μg·mL−1) | LOQs (μg·mL−1) |
|---|---|---|---|---|
| R2 | ||||
| Genipin | 0.9925 | 4.7 | 0.06 | 0.2 |
| Geniposide | 0.9938 | 3.9 | 0.03 | 0.1 |
| Lawsone | 0.9909 | 4.2 | 0.02 | 0.06 |
| (a) | Lawsone | (b) | Genipin | Geniposide | |||
|---|---|---|---|---|---|---|---|
| Sample Code | (μg·mL−1) | (μg·g−1) | Sample Code | (μg·mL−1) | (μg·g−1) | (μg·mL−1) | (μg·g−1) |
| HT-11 c | 20.5 | 7322.3 | JT-1 | 4.9 | 1681.5 | 3.6 | 1218.3 |
| HTD-1 | 1.9 | 831.5 | JT-2 | 5.5 | 1936.9 | 0.2 | 70.4 |
| HT-16 | 0.4 | 134.6 | JT-3 | 0.05 | 15.9 | 0.6 | 197.3 |
| HTD-2 | 1.4 | 600.5 | JT-4 | 15.8 | 5579.6 | 18.7 | 6586.0 |
| HTD-3 | 0.6 | 253.9 | JT-5 | 22.9 | 7310.7 | 1.9 | 607.9 |
| HD-1 | 0.9 | 330.9 | JT-6 | 20.5 | 5887.3 | 0.6 | 158.0 |
| HD-2 | 0.6 | 225.9 | HPT | - | - | - | - |
| HD-5 | 1.5 | 587.7 | |||||
| HD-7 | 1.6 | 543.8 | |||||
| RT (min) | Accurate Mass | Molecular Formula (Error mDa) | mSigma | Ionization Mode | Proposed Structure | Mostly Found in Samples |
|---|---|---|---|---|---|---|
| 1.10 | 325.12512 | C16H20O7 (3.06) | 32.9 | + | 11-Hydroxymonocerin | JT-2 |
| 1.53 | 195.03460 | C9H8O5 (4.63) | 13.7 | - | 3,4-Dihydroxyphenylpyruvic acid | JT-4, JT-1, JT-2 |
| 1.96 | 191.00304 | C9H4O5 (4.25) | 14.5 | - | Trimellitic anhydride | JT-4 |
| 2.70 | 219.05053 | C8H12O7 (0.49) | 6.5 | - | (−)-Threo-isodihomocitric acid | HPT |
| 4.09 | 259.05764 | C12H12O5 (0.08) | 16.6 | + | 5,7,8-Trimethoxycoumarin | HTD-1, HD-5, HTD-2, HTD-3 |
| 4.30 | 182.98801 | C7H4O6 (3.50) | 0.8 | - | Chelidonic acid | HT-14, HT-13, HT-12 |
| 4.38 | 137.01963 | C7H6O3 (4.70) | 8.5 | - | Salicylic acid | HT-4 |
| 7.61 | 280.26289 | C18H35NO2 (0.60) | 19.9 | + | Spiroxamine | JT-1, JT-4, JT-5 |
| 9.03 | 381.29836 | C21H42O4 (0.80) | 7.3 | + | Glyceryl monostearate | HT-14, HT-13, HT-16 |
| (a) | |||||||||
| RT (min) | Compound | Molecular Formula | Ion | Accurate Mass | mSigma | │Δm/z│ (mDa) | Ingredient Function | Annotation Sourced | Found in Samples |
| 1.10 | D-Sorbitol | C6H14O6 | C6H15O6+ | 183.08679 | 2.1 | 0.46 | Fragrance, humectant, perfuming, skin conditioning | B | JT-1, JT-2, JT-3, JT-4 |
| 1.11 | Lactitol | C12H24O11 | C12H25O11+ | 345.13904 | 15.9 | 0.11 | Humectant, skin conditioning | B | JT-1, JT-2, JT-3, JT-4 |
| 1.16 | Maltol | C6H6O3 | C6H7O3+ | 127.03752 | 12.6 | 0.23 | Fragrance, tonic | B | JT-1, JT-4, JT-2, JT-3 |
| 1.17 | Sucrose | C12H22O11 | C12H23O11+ | 343.12332 | 18.1 | 0.17 | Humectant, skin conditioning, soothing | A,B | JT-4, JT-1, JT-2, JT-3 |
| 1.54 | Glucose-6-phosphate | C6H13O9P | C6H14O9P+ | 261.03725 | 12.6 | 0.23 | - | B | JT-4, JT-2 |
| 1.93; 3.38 | Citric acid | C6H8O7 | [M+Na]+; C6H7O7− | 215.01668 | 8.4; 30.4 | 0.45; 1.48 | Buffering, chelating, fragrance | A,B; A | JT-4, JT-1, JT-2, JT-3; JT-1, JT-2, JT-4, JT-3 |
| 2.34 | 2,4,5-Trimethoxybenzoic acid | C10H12O5 | C10H13O5+ | 213.07649 | 2.3 | 0.76 | - | B | JT-4, JT-1, JT-3 |
| 2.44 | 3,4-Dihydrocoumarin | C9H9O2 | C9H10O2+ | 149.05937 | 6.8 | 0.34 | Fragrance, perfuming | B | JT-4, JT-3, JT-1, JT-2 |
| 2.52 | Sinapic acid | C11H12O5 | C11H13O5+ | 225.07641 | 10.6 | 0.66 | Skin conditioning | B | JT-4, JT-2, JT-1, JT-5, JT-6, JT-3 |
| 2.83 | 7-Methoxy-4-methyl-coumarin | C11H10O3 | C11H11O3+ | 191.07006 | 13.2 | 0.17 | Fragrance | B | JT-4, JT-5, JT-1, JT-6, JT-2, JT-3 |
| 3.10 | Picrotin | C15H18O7 | C15H19O7+ | 311.10928 | 17.7 | 3.25 | - | B | JT-6, JT-3, JT-1, JT-5, JT-4 |
| 3.38 | Scopoletin | C10H8O4 | C10H9O4+ | 193.04992 | 10.0 | 0.33 | - | B | JT-4, JT-1, JT-2, JT-3 |
| 3.43 | Phenylacetaldehyde | C8H8O | C8H9O+ | 121.06319 | 3.8 | 1.60 | Perfuming | B | JT-5, JT-6, JT-4, JT-2 |
| 3.77 | Geranic acid | C10H16O2 | C10H17O2+ | 169.12239 | 6.4 | 0.08 | Perfuming | B | JT-6, JT-5 |
| 4.70 | (DL)-Limonene | C10H16 | C10H17+ | 137.13132 | 10.9 | 1.16 | Deodorant, perfuming, solvent | A | JT-1, JT-3 |
| 4.75 | 4-Hydroxybenzoic acid | C7H6O3 | C7H7O3+ | 139.03788 | 0.4 | 1.09 | Fragrance, preservative | B | JT-5, JT-6 |
| 4.83 | Benzaldehyde | C7H6O | C7H7O+ | 107.04719 | 5.1 | 1.96 | Denaturant, fragrance, perfuming | A,B | JT-4, JT-5, JT-2 |
| 5.17 | N-Butylbenzenesulfonamide | C10H15NO2S | C10H16NO2S+ | 214.09072 | 7.8 | 1.17 | - | B | JT-3, JT-1, JT-2, JT-4, JT-5, JT-6 |
| 6.00 | Eudesmin | C22H26O6 | C22H27O6+ | 387.17996 | 10.9 | 0.71 | - | B | JT-5, JT-6, JT-2, JT-3, JT-4, JT-1 |
| 7.07 | Auraptene | C19H22O3 | C19H23O3+ | 299.16123 | 20.8 | 3.04 | - | B | JT-1, JT-2, JT-3, JT-6, JT-4, JT-5 |
| 7.19 | Cocamidopropyl betaine | C19H38N2O3 | C19H39N2O3+ | 343.29507 | 12.4 | 0.52 | Antistatic, cleansing, hair conditioning, skin conditioning, surfactant-foam boosting, viscosity controlling | B | JT-3, JT-4, JT-1, JT-2, JT-5, JT-6 |
| 7.37 | Phthalic anhydride | C8H4O3 | C8H5O3+ | 149.02269 | 3.3 | 0.63 | - | B | JT-1, JT-2, JT-3, JT-4, JT-5, JT-6 |
| 8.57 | Acetanilide | C8H9NO | C8H10NO+ | 136.07487 | 3.4 | 0.82 | Fragrance, perfuming | B | JT-3, JT-1, JT-2, JT-4, JT-5 |
| 8.85 | Triethanolamine | C6H15NO3 | C6H16NO3+ | 150.11214 | 13.3 | 0.33 | Buffering, fragrance, surfactant-cleansing, surfactant-emulsifying | B | JT-3, JT-6, JT-1, JT-2, JT-4, JT-5 |
| 8.96 | 2-Acetoxy-4-pentadecylbenzoic acid | C24H38O4 | C24H39O4+ | 391.28321 | 10.2 | 0.73 | - | B | JT-3, JT-1, JT-2, JT-4, JT-6, JT-5 |
| 8.97 | Dibutyl phthalate | C16H22O4 | C16H23O4+ | 279.15887 | 5.0 | 0.10 | Fragrance, perfuming, plasticiser, solvent | B | JT-1, JT-2, JT-6, JT-3, JT-4, JT-5 |
| (b) | |||||||||
| RT (min) | Compound | Molecular Formula | Ion | Accurate Mass | mSigma | │Δm/z│ (mDa) | Ingredient Function | Annotation Sourced | Found in Samples |
| 1.09 | D-Sorbitol | C6H14O6 | C6H15O6+ | 183.08686 | 1.7 | 0.52 | Fragrance, humectant, perfuming, skin conditioning | B | All HTD and HD, HT-16, HT-11 |
| 1.20 | Natural Red 4(CI 75470) | C22H20O13 | C22H19O13− | 491.08009 | 9.8 | 2.96 | Colorant, fragance | A | HT-3, HT-2, HTD-3, HT-6, HT-1, HT-4, HT-7, HTD-1, HTD-2, HT-16 |
| 1.94 | 4-Aminophenol | C6H7NO | C6H8NO+ | 110.05750 | 5.8 | 2.52 | Hair dyeing | B | HTD-3, HD-2, HD-3 |
| 2.48 | Indoline | C8H9N | C8H10N+ | 120.07877 | 2.3 | 2.01 | - | B | HTD-3, HTD-1 and all HD |
| 2.77 | Vanillic acid | C8H8O4 | C8H9O4+ | 169.04958 | 3.4 | 0.06 | - | B | All HTD and HD, HT-13, HT-2, HT-7 |
| 2.85 | Quinine | C20H24N2O2 | C20H25N2O2+ | 325.19068 | 20.1 | 0.38 | Denaturant, fragrance, hair conditioning | B | HD-1 |
| 3.06 | Syringic acid | C9H10O5 | C9H11O5+ | 199.06043 | 8.9 | 0.12 | Antioxidant | B | HD-2, HD-1, HTD-3, HD-3 |
| 3.37 | Coumarin | C9H6O2 | C9H7O2+ | 147.04354 | 5.0 | 0.52 | Perfuming | A, B | HTD-2, HTD-3, HTD-1, HD-7, HD-5, HT-16, HD-2, HD-4, HD-1, HD-3, HD-6, HT-11 |
| 3.53 | alpha-Santonin | C15H18O3 | C15H19O3+ | 247.13066 | 18.7 | 2.21 | - | B | All HTD and HD |
| 3.55 | Vitexin | C21H20O10 | C21H21O10+ | 433.11296 | 1.5 | 0.28 | - | B | HTD-1, HD-7, HD-5, HTD-2, HTD-3, HT-16, HD-4, HD-1 |
| 3.70 | Quercetin | C15H10O7 | C15H11O7+ | 303.05016 | 3.9 | 0.23 | Antioxidant, skin conditioning | A | All HD and HTD-1 |
| 3.87 | Ellagic acid | C14H6O8 | C14H7O8+ | 303.01326 | 14.0 | 0.28 | Skin conditioning | B | All HTD and HD (less HD-3, HD-6), HT-16 |
| 3.97 | Lawsonicin | C20H24O6 | [M+Na]+ | 383.14636 | 20.7 | 0.06 | - | A | HT-11, HT-16, HD-1, HD-7 |
| 4.07 | Methyl salicylate | C8H8O3 | C8H9O3+ | 153.05422 | 6.7 | 0.43 | Denaturant, oral care, perfuming, soothing | A | HT-13, HT-14, HT-1 |
| 4.19 | Salicylaldehyde | C7H6O2 | C7H7O2+ | 123.04385 | 4.0 | 0.20 | Perfuming | A,B | All |
| 4.25 | Hydroxycitronellal | C10H20O2 | [M+Na]+ | 195.13601 | 1.7 | 0.43 | Perfuming | A | HT-16, HT-14, HT-13, HT-15 |
| 4.26 | Hydroxyisohexyl 3- cyclohexene carboxaldehyde (HICC)/Lyral® | C13H22O2 | C13H23O2+ | 211.16982 | 11.9 | 0.59 | Fragrance, perfuming | A | All HD and HTD, HT-16 |
| 4.39 | 7-Methoxycoumarin | C10H8O3 | [M+Na]+ | 199.03715 | 6.8 | 0.43 | Fragrance | B | HD-6 |
| 4.77 | Tinnevellin glucoside | C20H24O9 | C20H25O9+ | 431.13127 | 13.4 | 0.02 | - | B | HD-6 |
| 4.85 | Luteolin | C15H10O6 | C15H11O6+ | 287.05472 | 3.3 | 0.21 | Skin conditioning | B | HT-16, HT-11, all HTD and HD |
| 4.89 | Hexyl cinnamal | C15H20O | C15H21O+ | 217.15919 | 8.6 | 0.61 | Perfuming | A | HD-6 |
| 5.29 | Daidzein | C15H10O4 | C15H11O4+ | 255.06527 | 6.0 | 0.08 | Skin conditioning-miscellaneous | B | HD-6 |
| 5.46 | Eugenol | C10H12O2 | C10H13O2+ | 165.09123 | 10.1 | 0.20 | Denaturant, perfuming, tonic | A,B | All |
| 5.86 | Glycitein | C16H12O5 | C16H13O5+ | 285.07564 | 7.5 | 0.11 | Antioxidant, skin conditioning, skin protecting | B | HD-6 |
| 6.00 | Eudesmin | C22H26O6 | C22H27O6+ | 387.18009 | 3.7 | 0.07 | - | B | All |
| 6.21 | Bisdemethoxy-curcumin | C19H16O4 | C19H17O4+ | 309.11250 | 4.3 | 0.37 | Antioxidant | B | HD-6 |
| 6.21 | Acid Orange 7(CI 15510) | C16H12N2O4S | C16H13N2O4S+ | 329.05884 | 11.5 | 0.15 | Hair dyeing | B | HT-10, HT-13, HT-15, HT-1, HT-2, HT-12 |
| 6.24 | Demethoxycurcumin | C20H18O5 | C20H19O5+ | 339.12249 | 5.1 | 0.21 | Antioxidant | A | HD-6 |
| 6.32 | 3-Phenoxybenzylalcohol | C13H12O2 | C13H13O2+ | 201.09201 | 8.1 | 1.00 | - | B | HD-6 |
| 6.53 | Geraniol | C10H18O | [M-H2O+H]+ | 137.13137 | 3.5 | 1.11 | Perfuming, tonic | A | HT-14 |
| 6.91 | Indigo | C16H10N2O2 | C16H11N2O2+ | 263.08092 | 6.0 | 0.56 | Hair dyeing | A,B | HD-3, HD-2, HTD-3 |
| 7.37 | Phthalic anhydride | C8H4O3 | C8H5O3+ | 149.02313 | 1.6 | 0.21 | - | B | All |
| 7.52 | Curcumin | C21H20O6 | C21H21O6+ | 369.13417 | 25.3 | 0.90 | Antioxidant, colorant | A,B | HD-6 |
| 7.68 | Alpha-santalol | C15H24O | [M+Na]+ | 243.17198 | 6.0 | 0.04 | Perfuming | A | HD-6 |
| 8.14 | Octocrylene | C24H27NO2 | [M+Na]+ | 384.19311 | 25.4 | 0.72 | Light stabilizer, UV filter | B | All |
| 8.50 | Avobenzone | C20H22O3 | [M+Na]+ | 333.14655 | 18.2 | 0.03 | Light stabilizer, UV filter | B | HT-13, HT-8, HT-14 |
| 8.57 | Acetanilide | C8H9NO | C8H10NO+ | 136.07526 | 4.8 | 0.43 | Fragrance, perfuming | B | All |
| 8.96 | Dibutyl phthalate | C16H22O4 | C16H23O4+ | 279.15916 | 4.8 | 0.08 | Fragrance, perfuming, plasticiser, solvent | B | All |
| 9.96 | Hexadecanolactone | C16H30O2 | C16H31O2+ | 255.23221 | 1.8 | 0.34 | Fragrance, perfuming | A | HT-16, HT-11 |
| (c) | |||||||||
| RT (min) | Compound | Molecular Formula | Ion | Accurate Mass | mSigma | │Δm/z│ (mDa) | Ingredient Function | Annotation Sourced | Found in Samples |
| 1.96 | Citric acid | C6H8O7 | C6H7O7− | 191.01883 | 9.2 | 0.89 | Buffering, chelating, fragrance | A,B | HPT |
| 3.61 | 3-Methyl-2-oxovaleric acid | C6H10O3 | C6H9O3− | 129.05420 | 5.0 | 1.38 | - | B | HPT |
| 7.14 | Dodecylbenzenesulfonic acid | C18H30O3S | C18H29O3S− | 325.18475 | 19.7 | 0.90 | Cleansing, surfactant-cleansing | B | HPT |
| 8.94 | Palmitic acid | C16H32O2 | C16H31O2− | 255.23250 | 16.2 | 0.47 | Skin conditioning-emollient, surfactant-emulsifying | B | HPT |
| 9.36 | Stearic acid | C18H36O2 | C18H35O2− | 283.26400 | 19.0 | 0.26 | Cleansing, emulsión stabilising, fragrance, refattig, surfactant-cleansing, surfactant-emulsifying | B | HPT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, L.; Garcia-Jares, C.; Lores, M. High-Resolution Mass Spectrometry for the Comprehensive Characterization of Plant-Pigment-Based Tattoos and Dyes Formulations. Cosmetics 2021, 8, 55. https://doi.org/10.3390/cosmetics8020055
Rubio L, Garcia-Jares C, Lores M. High-Resolution Mass Spectrometry for the Comprehensive Characterization of Plant-Pigment-Based Tattoos and Dyes Formulations. Cosmetics. 2021; 8(2):55. https://doi.org/10.3390/cosmetics8020055
Chicago/Turabian StyleRubio, Laura, Carmen Garcia-Jares, and Marta Lores. 2021. "High-Resolution Mass Spectrometry for the Comprehensive Characterization of Plant-Pigment-Based Tattoos and Dyes Formulations" Cosmetics 8, no. 2: 55. https://doi.org/10.3390/cosmetics8020055
APA StyleRubio, L., Garcia-Jares, C., & Lores, M. (2021). High-Resolution Mass Spectrometry for the Comprehensive Characterization of Plant-Pigment-Based Tattoos and Dyes Formulations. Cosmetics, 8(2), 55. https://doi.org/10.3390/cosmetics8020055