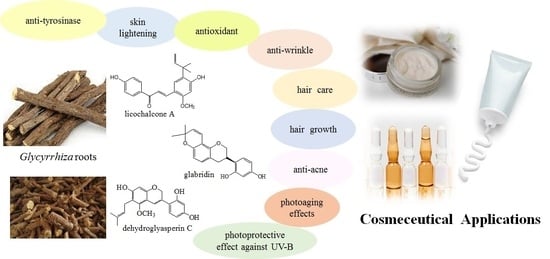
Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and Their Constituents as Active Cosmeceutical Ingredients
Abstract
:1. Introduction
2. Botanical Description
3. Chemistry of Glycyrrhiza
3.1. Flavonoids

3.2. Saponins
3.3. Polysaccharides
3.4. Species-Specific Markers for G. glabra, G. inflata, and G. uralensis
4. Skin Anti-Aging
4.1. Anti-Tyrosinase Activity and Hyperpigmentation Diseases
4.2. Skin Lightening Activity
4.3. Antiwrinkle Activity
5. Photoprotective Activity
5.1. Anti-Photoaging Effects
5.2. Photoprotective Effect against UV-B and Visible Radiation
5.3. Anti-Oxidant Effects
6. Hair Care
6.1. Hair Growth
6.2. General Hair Care and Dandruff
7. Anti-Acne Potential
Anti-Acne Activity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.H.; Zhang, L.; Wei, H.; Chen, H.D. Efficacy and safety of innovative cosmeceuticals. Clin. Dermatol. 2008, 26, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Batiha, G.E.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, J.; Yang, L.; Shen, S.; Li, P.; Yao, S.; Qu, H.; Li, J.; Yao, C.; Wei, W.; et al. Recent advances in chemical analysis of licorice (Gan-Cao). Fitoterapia 2021, 149, 104803. [Google Scholar] [CrossRef]
- Simayi, Z.; Rozi, P.; Yang, X.J.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.W.; Ma, S.J.; Askar, G.; Yadikar, N. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: Systematic review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef]
- Shakeri, A.; Masullo, M.; D’Urso, G.; Iranshahi, M.; Montoro, P.; Pizza, C.; Piacente, S. In depth chemical investigation of Glycyrrhiza triphylla Fisch roots guided by a preliminary HPLC-ESIMSn profiling. Food Chem. 2018, 248, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Kirmizibekmez, H.; Uysal, G.B.; Masullo, M.; Demirci, F.; Bagci, Y.; Kan, Y.; Piacente, S. Prenylated polyphenolic compounds from Glycyrrhiza iconica and their antimicrobial and antioxidant activities. Fitoterapia 2015, 103, 289–293. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, M.; Soundhari, C. Isolation and characterisation of mushroom tyrosinase and screening of herbal extracts for anti tyrosinase activity. Int. J. Chem. Tech. Res. 2017, 10, 1156–1167. [Google Scholar]
- Wang, C.; Chen, L.; Xu, C.; Shi, J.; Chen, S.; Tan, M.; Chen, J.; Zou, L.; Chen, C.; Liu, Z.; et al. A Comprehensive Review for Phytochemical, Pharmacological, and Biosynthesis Studies on Glycyrrhiza spp. Am. J. Chin. Med. 2020, 48, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A review on phytochemicals, pharmacological activities, drug interactions, and associated toxicities of licorice (Glycyrrhiza sp.). Food Front. 2021, 2, 449–485. [Google Scholar] [CrossRef]
- Li, F.; Liu, B.; Li, T.; Wu, Q.; Xu, Z.; Gu, Y.; Li, W.; Wang, P.; Ma, T.; Lei, H. Review of Constituents and Biological Activities of Triterpene Saponins from Glycyrrhizae Radix et Rhizoma and Its Solubilization Characteristics. Molecules 2020, 25, 3904. [Google Scholar] [CrossRef]
- Karkanis, A.; Martins, N.; Petropoulos, S.A.; Ferreira, I.C.F.R. Phytochemical composition, health effects, and crop management of liquorice (Glycyrrhiza glabra L.): A medicinal plant. Food Rev. Int. 2018, 34, 182–203. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Singh, D.C.; Pradhan, S.; Semwal, D.K. Comparative pharmacognostical & phytochemical study of Chakramard seed & Gomutra bhavit Chakramard seed. Eur. J. Biomed. Pharm. Sci. 2020, 7, 401–408. [Google Scholar]
- Sharma, V.; Katiyar, A.; Agrawal, R.C. Glycyrrhiza glabra: Chemistry and Pharmacological Activity. Sweeten. Pharmacol. Biotechnol. Appl. 2017, 87–100. [Google Scholar] [CrossRef]
- Simmler, C.; Pauli, G.F.; Chen, S.-N. Phytochemistry and biological properties of glabridin. Fitoterapia 2013, 90, 160–184. [Google Scholar] [CrossRef] [Green Version]
- Masullo, M.; Pizza, C.; Piacente, S. Oleanane derivatives for pharmaceutical use: A patent review (2000–2016). Expert. Opin. Ther. Pat. 2017, 27, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; Maldini, M.; Russo, M.; Postorino, S.; Piacente, S.; Pizza, C. Metabolic profiling of roots of liquorice (Glycyrrhiza glabra) from different geographical areas by ESI/MS/MS and determination of major metabolites by LC-ESI/MS and LC-ESI/MS/MS. J. Pharm. Biomed. 2011, 54, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Qiao, X.; Chen, K.; Wang, Y.; Ji, S.; Feng, J.; Li, K.; Lin, Y.; Ye, M. Biosynthesis-Based Quantitative Analysis of 151 Secondary Metabolites of Licorice To Differentiate Medicinal Glycyrrhiza Species and Their Hybrids. Anal. Chem. 2017, 89, 3146–3153. [Google Scholar] [CrossRef]
- Xiang, C.; Qiao, X.; Ye, M.; Guo, D.A. Classification and distribution analysis of components in Glycyrrhiza using licorice compounds database. Acta Pharm. Sin. 2012, 47, 1023–1030. [Google Scholar]
- Jiang, Z.Z.; Wang, Y.F.; Zheng, Y.F.; Yang, J.; Zhang, L. Ultra high performance liquid chromatography coupled with triple quadrupole mass spectrometry and chemometric analysis of licorice based on the simultaneous determination of saponins and flavonoids. J. Sep. Sci. 2016, 39, 2928–2940. [Google Scholar] [CrossRef]
- Baba, M.; Fukuda, E.; Uesawa, Y.; Kai, H.; Matsuno, K.; Okada, Y. Application of Mixture Analysis to Crude Materials from Natural Resources (V) [1]: Discrimination of Glycyrrhiza uralensis and G. glabra by El mass spectrometry. Nat. Prod. Commun. 2017, 12, 27–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef]
- Simmler, C.; Anderson, J.R.; Gauthier, L.; Lankin, D.C.; McAlpine, J.B.; Chen, S.N.; Pauli, G.F. Metabolite Profiling and Classification of DNA-Authenticated Licorice Botanicals. J. Nat. Prod. 2015, 78, 2007–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, R.; Nakano, F.; Ohno, H.; Murakami, T.; Okada, Y.; Shirataki, Y. Distinguishing Glycyrrhiza species using NMR-based Metabolomics. Nat. Prod. Comm. 2018, 13, 71–73. [Google Scholar] [CrossRef] [Green Version]
- Vijayalakshmi, U.; Shourie, A. Evaluation of different methods for the extraction of antioxidant phenolic compounds from Glycyrrhiza glabra roots. World J. Pharm. Res. 2015, 4, 1524–1537. [Google Scholar]
- Tian, M.; Yan, H.; Row, K.H. Extraction of Glycyrrhizic Acid and Glabridin from Licorice. Int. J. Mol. Sci. 2008, 9, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Guo, Z.; Xiao, T.; Liu, H.; Su, G.; Zhao, Y. Enrichment of total flavones and licochalcone A from licorice residues and its hypoglycemic activity. J. Chromatogr. B 2019, 1114–1115, 134–145. [Google Scholar] [CrossRef]
- Campa, M.; Baron, E. Anti-aging Effects of Select Botanicals: Scientific Evidence and Current Trends. Cosmetics 2018, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an Important Source of Antioxidants and Their Applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Mahendra, C.K.; Tan, L.T.H.; Mahendra, C.K.; Ser, H.-L.; Pusparajah, P.; Htar, T.T.; Chuah, L.-H.; Yap, W.H.; Tang, S.Y.; Ming, L.C.; et al. The Potential of Sky Fruit as an Anti-Aging and Wound Healing Cosmeceutical Agent. Cosmetics 2021, 8, 79. [Google Scholar] [CrossRef]
- Kirubakaran, N.; Thiyagarajan; Mahendra, C.; Varma, S.; Kumar, V.; Tripathi, V.; Babu. Design and development of emulsion-based clear complexion skin whitening cream using Glycyrrhiza glabra (Licorice) root extract and Vateria indica (white dammar) bark extract by skin melanin inhibitory pathway. Int. J. Pharm. Sci. Res. 2017, 8, 1210–1219. [Google Scholar] [CrossRef]
- Ruchi, G.; Rajiv, S.; Archana, P.; Yashu, C.; Neelesh, M. Review on antityrosinase activity of some Indian medicinal plants and their phytoconstituents. J. Drug Deliv. Ther. 2020, 10, 199–204. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Opperman, L.; De Kock, M.; Klaasen, J.; Rahiman, F. Tyrosinase and Melanogenesis Inhibition by Indigenous African Plants: A Review. Cosmetics 2020, 7, 60. [Google Scholar] [CrossRef]
- Uto, T.; Ohta, T.; Yamashita, A.; Fujii, S.; Shoyama, Y. Liquiritin and liquiritigenin induce melanogenesis via enhancement of p38 and PKA signaling pathways. Medicines 2019, 6, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.-L.; Aligiannis, N. Anti-melanogenic properties of Greek plants. A novel depigmenting agent from Morus alba wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Gao, J. The Use of Botanical Extracts as Topical Skin-Lightening Agents for the Improvement of Skin Pigmentation Disorders. J. Investig. Dermatol. Symp. Proc. 2008, 13, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory mechanisms of glabridin on tyrosinase. Spectrochim. Acta Part A 2016, 168, 111–117. [Google Scholar] [CrossRef]
- Hespeler, D.; Kaltenbach, J.; Pyo, S.M. Glabridin smartPearls—Silica selection, production, amorphous stability and enhanced solubility. Int. J. Pharm. 2019, 561, 228–235. [Google Scholar] [CrossRef]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, M.; Zhao, T.; Feng, M.; Chen, H.; Zhuang, M.; Lin, L. Bioactive profiles, antioxidant activities, nitrite scavenging capacities and protective effects on H2O2-injured PC12 cells of Glycyrrhiza Glabra L. leaf and root extracts. Molecules 2014, 19, 9101–9113. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Kuang, Y.; Li, K.; Wang, S.; Song, W.; Qiao, X.; Sabir, G.; Ye, M. Screening for bioactive natural products from a 67-compound library of Glycyrrhiza inflata. Bioorg. Med. Chem. 2017, 25, 3706–3713. [Google Scholar] [CrossRef]
- Liu, L.; Shi, S.; Chen, X.; Peng, M. Analysis of tyrosinase binders from Glycyrrhiza uralensis root: Evaluation and comparison of tyrosinase immobilized magnetic fishing-HPLC and reverse ultrafiltration-HPLC. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 932, 19–25. [Google Scholar] [CrossRef]
- Lim, J.W.; Ha, J.H.; Jeong, Y.J.; Park, S.N. Anti-melanogenesis effect of dehydroglyasperin C through the downregulation of MITF via the reduction of intracellular cAMP and acceleration of ERK activation in B16F1 melanoma cells. Pharmacol. Rep. 2018, 70, 930–935. [Google Scholar] [CrossRef]
- Maddaleno, A.S.; Camargo, J.; Mitjans, M.; Vinardell, M.P. Melanogenesis and Melasma Treatment. Cosmetics 2021, 8, 82. [Google Scholar] [CrossRef]
- Amer, M.; Metwalli, M. Topical liquiritin improves melasma. Int. J. Dermatol. 2000, 39, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Alexis, A.F.; Blackcloud, P. Natural ingredients for darker skin types: Growing options for hyperpigmentation. J. Drugs Dermatol. 2013, 12, s123–s127. [Google Scholar] [PubMed]
- Ishi, H.S.; Pawar, S.P.; Patil, S.T. A research: Design, development and evaluation of herbal skin lightening cream. World J. Pharm. Pharm. Sci. 2017, 6, 992–1003. [Google Scholar] [CrossRef]
- Parashar, B.; Sharma, P.; Kabra, A. Formulation and evaluation of polyherbal face cream. Int. Pharm. Sci. 2013, 3, 63–68. [Google Scholar]
- Kim, H.J.; Seo, S.H.; Lee, B.-g.; Lee, Y.S. Identification of tyrosinase inhibitors from Glycyrrhiza uralensis. Planta Med. 2005, 71, 785–787. [Google Scholar] [CrossRef]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive Constituents of Glycyrrhiza uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in Skincare-The Fascinating World of Skin-Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation. Cosmetics 2021, 8, 66. [Google Scholar] [CrossRef]
- Ryu, H.W.; Cho, J.K.; Curtis-Long, M.J.; Yuk, H.J.; Kim, Y.S.; Jung, S.; Kim, Y.S.; Lee, B.W.; Park, K.H. α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry 2011, 72, 2148–2154. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Hooda, R. Antiwrinkle herbal drugs—An update. J. Pharmacogn. Phytochem. 2015, 4, 277–281. [Google Scholar]
- Ciganovic, P.; Jakimiuk, K.; Tomczyk, M.; Koncic, M.Z. Glycerolic licorice extracts as active cosmeceutical ingredients: Extraction optimization, chemical characterization, and biological activity. Antioxidants 2019, 8, 445. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.; Sahoo, M.; Roy, S.D.; Dasgupta, R.K. Anti-ageing natural herbs: A systemic review. Indian Res. J. Pharm. Sci. 2018, 5, 1589–1598. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.-T.; Jang, Y.; Myung, S.-W.; Lee, J.M.; Kim, H.-S.; Ko, S.-C.; Lee, S.-H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]
- Dey, S.; Deepak, M.; Setty, M.; D’Souza, P.; Agarwal, A.; Sangli, G.K. Bioactive caffeic acid esters from Glycyrrhiza glabra. Nat. Prod. Res. 2009, 23, 1657–1663. [Google Scholar] [CrossRef]
- Offord, E.A.; Gautier, J.-C.; Avanti, O.; Scaletta, C.; Runge, F.; Kramer, K.; Applegate, L.A. Photoprotective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radical Biol. Med. 2002, 32, 1293–1303. [Google Scholar] [CrossRef]
- F’Guyer, S.; Afaq, F.; Mukhtar, H. Photochemoprevention of skin cancer by botanical agents. Photodermatol. Photoimmunol. Photomed. 2003, 19, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hasegawa, J.; Asamitsu, K.; Okamoto, T. Prevention of the ultraviolet B-mediated skin photoaging by a nuclear factor κB inhibitor, parthenolide. J. Pharmacol. Exp. Ther. 2005, 315, 624–630. [Google Scholar] [CrossRef] [Green Version]
- Afnan, Q.; Adil, M.D.; Nissar-Ul, A.; Rafiq, A.R.; Amir, H.F.; Kaiser, P.; Gupta, V.K.; Vishwakarma, R.; Tasduq, S.A. Glycyrrhizic acid (GA), a triterpenoid saponin glycoside alleviates ultraviolet-B irradiation-induced photoaging in human dermal fibroblasts. Phytomedicine 2012, 19, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.H.; Park, Y.M.; Ha, J.H.; Jeong, Y.J.; Park, S.N. The effect of dehydroglyasperin C on UVB-mediated MMPs expression in human HaCaT cells. Pharmacol. Rep. 2017, 69, 1224–1231. [Google Scholar] [CrossRef]
- Puri, A.; Kaur, A.; Raza, K.; Goindi, S.; Katare, O.P. Development and evaluation of topical microemulsion of dibenzoylmethane for treatment of UV induced photoaging. J. Drug Delivery Sci. Technol. 2017, 37, 1–12. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeon, H.Y.; Kim, S.K.; Lee, H.K.; Kim, B.J. The beneficial effects of an oriental herbal complex supplement on women’s hair and scalp conditions: A 24-week, randomized, double-blind, placebo-controlled study. J. Food Nutr. Res. 2017, 5, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Mann, T.; Eggers, K.; Rippke, F.; Tesch, M.; Buerger, A.; Darvin, M.E.; Schanzer, S.; Meinke, M.C.; Lademann, J.; Kolbe, L. High-energy visible light at ambient doses and intensities induces oxidative stress of skin-Protective effects of the antioxidant and Nrf2 inducer Licochalcone A in vitro and in vivo. Photodermatol. Photoimmunol. Photomed. 2020, 36, 135–144. [Google Scholar] [CrossRef]
- Liu, J.; Luo, L.; Zhang, H.; Jia, B.; Lu, J.; Li, P.; Chen, J. Rapid screening for novel antioxidants in Glycyrrhiza inflata using high-resolution peak fractionation. J. Funct. Foods 2015, 16, 40–49. [Google Scholar] [CrossRef]
- Kolbe, L.; Immeyer, J.; Batzer, J.; Wensorra, U.; tom Dieck, K.; Mundt, C.; Wolber, R.; Staeb, F.; Schoenrock, U.; Ceilley, R.I.; et al. Anti-inflammatory efficacy of Licochalcone A: Correlation of clinical potency and in vitro effects. Arch. Dermatol. Res. 2006, 298, 23–30. [Google Scholar] [CrossRef]
- Weber, T.M.; Ceilley, R.I.; Buerger, A.; Kolbe, L.; Trookman, N.S.; Rizer, R.L.; Schoelermann, A. Skin tolerance, efficacy, and quality of life of patients with red facial skin using a skin care regimen containing Licochalcone A. J. Cosmet. Dermatol. 2006, 5, 227–232. [Google Scholar] [CrossRef]
- Afreen, F.; Zobayed, S.M.A.; Kozai, T. Melatonin in Glycyrrhiza uralensis: Response of plant roots to spectral quality of light and UV-B radiation. J. Pineal Res. 2006, 41, 108–115. [Google Scholar] [CrossRef]
- Sharma, S.; Vasudeva, N.; Singh, S.; Das, S. Formulation and evaluation of post laser herbal cream. Int. J. Green Pharm. 2018, 12, 90–94. [Google Scholar]
- González-Minero, F.J.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Rackova, L.; Jancinova, V.; Petrikova, M.; Drabikova, K.; Nosal, R.; Stefek, M.; Kostalova, D.; Pronayova, N.; Kovacova, M. Mechanism of anti-inflammatory action of liquorice extract and glycyrrhizin. Nat. Prod. Res. 2007, 21, 1234–1241. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Duenas, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds and antioxidant properties of Glycyrrhiza glabra L. rhizomes and roots. RSC Adv. 2015, 5, 26991–26997. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, H.; Ishikawa, H.; Mizutani, K.; Tamura, Y.; Kinoshita, T. Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata. Bioorg. Med. Chem. 1998, 6, 339–347. [Google Scholar] [CrossRef]
- Biondi, D.M.; Rocco, C.; Ruberto, G. New dihydrostilbene derivatives from the leaves of Glycyrrhiza glabra and evaluation of their antioxidant activity. J. Nat. Prod. 2003, 66, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Morteza-Semnani, K.; Saeedi, M.; Shahnavaz, B. Comparison of antioxidant activity of extract from roots of licorice (Glycyrrhiza glabra L.) to commercial antioxidants in 2% hydroquinone cream. J. Cosmet. Sci. 2003, 54, 551–558. [Google Scholar]
- Niziol-Lukaszewska, Z.; Bujak, T. Saponins as Natural Raw Materials for Increasing the Safety of Bodywash Cosmetic Use. J. Surfactants Deterg. 2018, 21, 767–776. [Google Scholar] [CrossRef]
- Rozi, P.; Abuduwaili, A.; Ma, S.; Bao, X.; Xu, H.; Zhu, J.; Yadikar, N.; Wang, J.; Yang, X.; Yili, A. Isolationscharacterizations and bioactivities of polysaccharides from the seeds of three species Glycyrrhiza. Int. J. Biol. Macromol. 2020, 145, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Bhide, M.M.; Nitave, S.A. Formulation and evaluation of polyherbal cosmetic cream. World J. Pharm. Pharm. Sci. 2016, 5, 1527–1536. [Google Scholar]
- Shivakant, S.; Kaushelendra, M.; Singhai, A.K.; Purnima, S.; Nayak, S. Hair care: From natural ingredients. Eur. J. Biomed. Pharm. Sci. 2020, 7, 401–409. [Google Scholar]
- Utami, S.M.; Djajadisastra, J.; Saputri, F.C. Using hair growth activity, physical stability, and safety tests to study hair tonics containing ethanol extract of licorice (Glycyrrhiza glabra Linn.). Int. J. Appl. Pharm. 2017, 9, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Murthy, A.R.; Sharma, G. Quality assessment of Yashtimadhukadi taila: Ayurveda remedy for Khalitya (hair fall). Int. J. Green Pharm. 2018, 12, S722–S727. [Google Scholar]
- Bhavani, M.D.; Sridurga, C. Analytical study of Tiladi Taila—A good remedy for hair fall. World J. Pharm. Res. 2017, 6, 1586–1593. [Google Scholar] [CrossRef]
- Huang, B.; Kang, B.-G.; Wang, Z.; Lim, S.S. Effect of ethanol extract of plant mixture on hair regeneration in human dermal papilla cells and C57BL/6J mice. J. Med. Plants Res. 2015, 9, 1103–1110. [Google Scholar] [CrossRef]
- Schweiger, D.; Rippke, F.; Drescher, P.; Braren, S.; Luettke, J.; Filbry, A.; Max, H. Highly efficient rinse-off/leave-on scalp care treatments to reduce moderate to severe dandruff. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.C.; Kumari, R.; Iqbal, Z.; Rizvi, M.M.A.; Yadav, J.P. Synthesis, characterization, comparative antidandruff efficacy and cytotoxicity studies of biosynthesized silver nanoparticles by using Glycyrrhiza glabra root. Adv. Sci. Eng. Med. 2020, 12, 156–162. [Google Scholar] [CrossRef]
- Keshri, P.; Khare, E. Antiacne synergistic herbal face wash: Formulation and evaluation. World J. Pharm. Res. 2020, 9, 1899–1907. [Google Scholar] [CrossRef]
- Nakyai, W.; Pabuprapap, W.; Sroimee, W.; Ajavakom, V.; Yingyongnarongkul, B.-e.; Suksamrarn, A. Anti-Acne Vulgaris Potential of the Ethanolic Extract of Mesua ferrea L. Flowers. Cosmetics 2021, 8, 107. [Google Scholar] [CrossRef]
- Nam, C.; Kim, S.; Sim, Y.; Chang, I. Anti-acne effects of Oriental herb extracts: A novel screening method to select anti-acne agents. Skin Pharmacol. Physiol. 2003, 16, 84–90. [Google Scholar] [CrossRef]
- Reuter, J.; Merfort, I.; Schempp, C.M. Botanicals in dermatology: An evidence-based review. Am. J. Clin. Dermatol. 2010, 11, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Shama, S.N.; Joy, J.M.; Reddy, B.S.; Roja, C. Formulation and evaluation of herbal anti-acne moisturizer. Pak. J. Pharm. Sci. 2012, 25, 867–870. [Google Scholar] [PubMed]
- Nand, P.; Drabu, S.; Gupta, R.K.; Bhatnagar, A.; Ali, R. In vitro and in vivo assessment of polyherbal topical gel formulation for the treatment of acne vulgaris. Int. J. Drug Deliv. 2012, 4, 434–442. [Google Scholar]
- Nand, P.; Drabu, S.; Gupta, R.K. Phytochemical and antimicrobial screening of medicinal plants for the treatment of acne. Indian J. Nat. Prod. Resour. 2012, 3, 28–32. [Google Scholar]
- Sravani, L.V.; Rao, C.B.; Babu, R.K.D. Anti-acne activity of lipido-sterolic extract of serenoa repens and hydro-alcoholic extract of Gycyrrhiza glabra in Syrian hamster ear model. Indo Am. J. Pharm. Sci. 2017, 4, 2641–2650. [Google Scholar] [CrossRef]
- Lakshmi, J.N.; Supriya, P.; Soumya, P.L.; Vandana, P.; Babu, A.N. Pharmacological evaluation of liquorice for various dermatological disorders in mice. Int. J. Pharm. Biol. Sci. 2019, 9, 1253–1259. [Google Scholar] [CrossRef]
- Rekha, N.; Austin, A. In vitro study on the anti—Acne property of ayurvedic soap and body wash. World J. Pharm. Res. 2017, 6, 865–870. [Google Scholar] [CrossRef]
- Koli, D.S.; Mane, A.N.; Kumbhar, V.B.; Shaha, K.S. Formulation & evaluation of herbal anti-acne face wash. World J. Pharm. Pharm. Sci. 2016, 5, 2001–2007. [Google Scholar] [CrossRef]
- Wagh, V.; Shaikh, S.; Maynale, S.S.; Mhaske, N. Preparation and evaluation of marigold, liquorice and corange peel extract containing herbal face wash. World J. Pharm. Res. 2015, 4, 1808–1812. [Google Scholar]
- Shivatare, R.S.; Kewatkar, S.M.; Lohakare, P.; Bhutale, N.; Musale, R.; Choudhary, D.; Ganu, G.; Nagore, D.H. Anti-inflammatory, anti-oxidant and anti-microbial properties of polyherbal formulation in acne treatment. Int. J. Pharm. Sci. Rev. Res. 2020, 62, 73–77. [Google Scholar]
- Yang, G.; Lee, H.E.; Yeon, S.H.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Zouboulis, C.C.; Han, S.-H.; Lee, J.-H.; Lee, J.Y. Licochalcone A attenuates acne symptoms mediated by suppression of NLRP3 inflammasome. Phytother. Res. 2018, 32, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Rameshrad, M.; Hosseinzadeh, H. Toxicological Effects of Glycyrrhiza glabra (Licorice): A Review. Phytother. Res. 2017, 31, 1635–1650. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerulli, A.; Masullo, M.; Montoro, P.; Piacente, S. Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and Their Constituents as Active Cosmeceutical Ingredients. Cosmetics 2022, 9, 7. https://doi.org/10.3390/cosmetics9010007
Cerulli A, Masullo M, Montoro P, Piacente S. Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and Their Constituents as Active Cosmeceutical Ingredients. Cosmetics. 2022; 9(1):7. https://doi.org/10.3390/cosmetics9010007
Chicago/Turabian StyleCerulli, Antonietta, Milena Masullo, Paola Montoro, and Sonia Piacente. 2022. "Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and Their Constituents as Active Cosmeceutical Ingredients" Cosmetics 9, no. 1: 7. https://doi.org/10.3390/cosmetics9010007
APA StyleCerulli, A., Masullo, M., Montoro, P., & Piacente, S. (2022). Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and Their Constituents as Active Cosmeceutical Ingredients. Cosmetics, 9(1), 7. https://doi.org/10.3390/cosmetics9010007