Fibroblasts Collagen Production and Histological Alterations in Hereditary Gingival Fibromatosis
Abstract
:1. Introduction
2. Case Report
3. Methods and Results
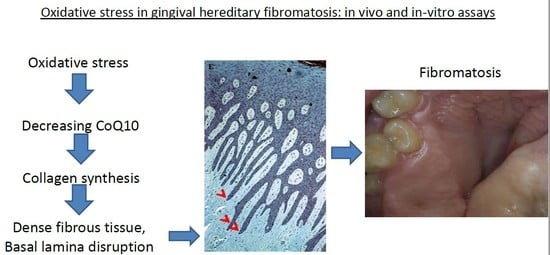
3.1. Histology
3.2. Fibroblast Culture
3.3. Collagen Assay
3.4. CoQ10 Level Determination
3.5. Lipid Peroxidation
3.6. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Breen, G.H.; Addante, R.; Black, C.C. Early onset of hereditary gingival fibromatosis in a 28-month-old. Pediatr Dent. 2009, 3, 286–288. [Google Scholar]
- Bozzo, L.; Machado, M.A.; de Almeida, O.P.; Lopes, M.A.; Coletta, R.D. Hereditary gingival fibromatosis: Report of three cases. J. Clin. Pediatr. Dent. 2000, 25, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Witkop, C.J. Heterogeneity in inherited dental traits, gingival fibromatosis and amelogenesis imperfecta. South Med. J. 1971, 64, 16–25. [Google Scholar] [CrossRef]
- Bozzo, L.; de Almedia, O.P.; Scully, C.; Aldred, M.J. Hereditary gingival fibromatosis. Report of an extensive four-generation pedigree. Oral. Surg. Oral. Med. Oral. Pathol. 1994, 78, 452–454. [Google Scholar] [CrossRef]
- Hart, T.C.; Pallos, D.; Bowden, D.W.; Bolyard, J.; Pettenati, M.J.; Cortelli, J.R. Genetic linkage of hereditary gingival fibromatosis to chromosome 2p21. Am. J. Hum. Genet 1998, 62, 876–883. [Google Scholar] [CrossRef]
- Gawron, K.; Łazarz-Bartyzel, K.; Potempa, J.; Chomyszyn-Gajewska, M. Gingival fibromatosis: Clinical, molecular and therapeutic issues. Orphanet J. Rare Dis. 2016, 11, 9. [Google Scholar] [CrossRef]
- Kataoka, M.; Kido, J.; Shinohara, Y.; Nagata, T. Drug-induced gingival overgrowth—A review. Biol. Pharm. Bull. 2005, 28, 1817–1821. [Google Scholar] [CrossRef]
- Araujo, C.S.; Graner, E.; Almeida, O.P.; Sauk, J.J.; Coletta, R.D. Histomorphometric characteristics and expression of epidermal growth factor and its receptor by epithelial cells of normal gingiva and hereditary gingival fibromatosis. J. Periodontal. Res. 2003, 38, 237–241. [Google Scholar] [CrossRef]
- Farrer-Brown, G.; Lucas, R.B.; Winstock, D. Familial gingival fibromatosis: An unusual pathology. J. Oral Pathol. 1972, 1, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Günhan, O.; Gardner, D.G.; Bostanci, H.; Günhan, M. Familial gingival fibromatosis with unusual histologic findings. J. Periodontol. 1995, 66, 1008–1011. [Google Scholar] [CrossRef]
- Coletta, R.D.; Graner, E. Hereditary gingival fibromatosis: A systematic review. J. Periodontol. 2006, 77, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Boyer, B.; Vallés, A.M.; Edme, N. Induction and regulation of epithelial-mesenchymal transitions. Biochem. Pharmacol. 2000, 60, 1091–1099. [Google Scholar] [CrossRef]
- Battino, M.; Bullon, P.; Wilson, M.; Newman, H. Oxidative injury and inflammatory periodontal diseases: The challenge of anti-oxidants to free radicals and reactive oxygen species. Crit. Rev. Oral. Biol. Med. 1999, 10, 458–476. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Newman, H.N.; Battino, M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: A shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol 2000 2017, 64, 139–153. [Google Scholar] [CrossRef]
- Becerik, S.; Celec, P.; Gürkan, A.; Öztürk, V.Ö.; Kamodyova, N.; Atilla, G.; Emingil, G. Gingival crevicular fluid and plasma levels of Transglutaminase-2 and oxidative stress markers in Cyclosporin A-Induced gingival overgrowth. J. Periodontol. 2016, 87, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ramer, M.; Marrone, J.; Stahl, B.; Burakoff, R. Hereditary gingival fibromatosis: Identification, treatment, control. J. Am. Dent. Assoc. 1996, 127, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, L.P.; Campos, V.; Moliterno, L.F.; Ribeiro, D.P.; Sampaio, R.K. Hereditary gingival fibromatosis: Review of the literature and a case report. Quintessence Int. 2000, 31, 415–418. [Google Scholar]
- Pego, S.P.; Coletta, R.D.; Mendes, D.C.; de Faria, P.R.; Melo-Filho, M.R.; Alves, L.R.; Martelli-Júnior, H. Hereditary gingival fibromatosis: Clinical and ultrastructural features of a new family. Med. Oral. Patol. Oral. Cir. Bucal. 2015, 20, e150–e155. [Google Scholar] [CrossRef] [PubMed]
- Tipton, D.A.; Howell, K.J.; Dabbous, M.K. Increased proliferation, collagen, and fibronectin production by hereditary gingival fibromatosis fibroblasts. J. Periodontol. 1997, 68, 524–530. [Google Scholar] [CrossRef]
- Fu, M.M.; Chin, Y.T.; Fu, E.; Chiu, H.C.; Wang, L.Y.; Chiang, C.Y.; Tu, H.P. Role of transforming growth factor-beta1 in cyclosporine-induced epithelial-to-mesenchymal transition in gingival epithelium. J. Periodontol. 2015, 86, 120–128. [Google Scholar] [CrossRef]
- Kantarci, A.; Nseir, Z.; Kim, Y.S.; Sume, S.S.; Trackman, P.C. Loss of basement membrane integrity in human gingival overgrowth. J. Dent. Res. 2011, 90, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Gawron, K.; Łazarz-Bartyzel, K.; Fertala, A.; Plakwicz, P.; Potempa, J.; Chomyszyn-Gajewska, M. Gingival fibromatosis with significant de novo formation of fibrotic tissue and a high rate of recurrence. Am. J. Case Rep. 2016, 17, 655–659. [Google Scholar] [CrossRef]
- Coletta, R.D.; Almeida, O.P.; Reynolds, M.A.; Sauk, J.J. Alteration in expression of MMP-1 and MMP-2 but not TIMP-1 and TIMP-2 in hereditary gingival fibromatosis is mediated by TGF-beta 1 autocrine stimulation. J. Periodontal. Res. 1999, 34, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Da Ros Gonçalves, L.; Oliveira, G.A.; Borojevic, R.; Otazu, I.B.; Feres-Filho, E.J. Expression of metalloproteinases and their tissue inhibitors in gingiva affected by hereditary gingival fibromatosis: Analysis of three cases within a family. J. Periodontal. Res. 2009, 44, 714–717. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Q.; Fang, G.; Li, B.S.; Wu, D.B.; Guo, W.J.; Hong, S.S.; Hong, L. Collagen metabolic disorder induced by oxidative stress in human uterosacral ligament-derived fibroblasts: A possible pathophysiological mechanism in pelvic organ prolapse. Mol. Med. Rep. 2016, 13, 2999–3008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Wei, C.C.; Powell, P.C.; Bradley, W.E.; Collawn, J.F.; Dell’Italia, L.J. Volume overload induces autophagic degradation of procollagen in cardiac fibroblasts. J. Mol. Cell. Cardiol. 2015, 89, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman-Malo, L.; Bullon, B.; de Miguel, M.; Bullon, P. Fibroblasts Collagen Production and Histological Alterations in Hereditary Gingival Fibromatosis. Diseases 2019, 7, 39. https://doi.org/10.3390/diseases7020039
Roman-Malo L, Bullon B, de Miguel M, Bullon P. Fibroblasts Collagen Production and Histological Alterations in Hereditary Gingival Fibromatosis. Diseases. 2019; 7(2):39. https://doi.org/10.3390/diseases7020039
Chicago/Turabian StyleRoman-Malo, Lourdes, Beatriz Bullon, Manuel de Miguel, and Pedro Bullon. 2019. "Fibroblasts Collagen Production and Histological Alterations in Hereditary Gingival Fibromatosis" Diseases 7, no. 2: 39. https://doi.org/10.3390/diseases7020039
APA StyleRoman-Malo, L., Bullon, B., de Miguel, M., & Bullon, P. (2019). Fibroblasts Collagen Production and Histological Alterations in Hereditary Gingival Fibromatosis. Diseases, 7(2), 39. https://doi.org/10.3390/diseases7020039