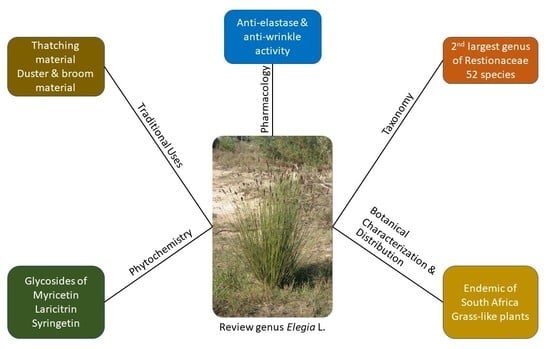
Traditional Uses, Phytochemistry, and Pharmacology of Elegia Species: A Review
Abstract
:1. Introduction
2. Materials and Methods
3. Botanical Characterization and Distribution
| Botanical Name | Synonyms (Previous Botanical Name) |
|---|---|
| E. acockii (Pillans) Moline and H.P.Linder | Chondropetalum acockii Pillans |
| E. aggregata (Mast.) Moline and H.P.Linder | C. aggregatum (Mast) Pillans Dovea aggregata Mast. |
| E. altigena Pillans | - |
| E. amoena Pillans | - |
| E. asperiflora (Nees) Kunth | E. ciliata Mast. E. glauca Mast. E. lacerata Pillans Restio asperiflorus Nees |
| E. atratiflora Esterh. | - |
| E. caespitosa Esterh. | - |
| E. capensis (Burm.f.) Schelpe | E. verticillaris (L.f.) Kunth Equisetum capense Burm. f. Restio verticillaris L.f. |
| E. coleura Nees ex Mast. | E. exilis Mast. |
| E. cuspidata Mast. | - |
| E. decipiens (Esterhuysen) Moline and H.P.Linder | Chondropetalum decipiens Esterhuysen |
| E. deustum (Rottb.) Kunth | Chondropetalum deustum Rottb. Elegia deusta (Rottb.) Kunth Restio chondropetalum Nees |
| E. dregeana Kunth | - |
| E. ebracteata (Kunth) Moline and H.P.Linder | Chondropetalum ebracteatum (Kunth) Pillans Dovea ebracteata Kunth |
| E. elephantina H.P.Linder | - |
| E. equisetacea Mast. | E. equisetacea (Mast.) Mast. E. propinqua (Nees) Kunth var. equisetacea Mast |
| E. esterhuyseniae Pillans | - |
| E. extensa Pillans | - |
| E. fastigiata Mast. | - |
| E. fenestrata Pillans | - |
| E. filacea Mast. | E. gracilis N.E.Br. E. parviflora Pillans E. parviflora (Thunb.) Kunth E. parviflora Pillans var. filacea (Mast.) Pillans E. rehmanni Mast |
| E. fistulosa Kunth | - |
| E. fucata Esterhuysen | - |
| E. galpinii N.E.Br. | - |
| E. grandis (Nees) Kunth | Lamprocaulis grandis (Nees) Mast. Restio grandis Spreng. Ex Nees |
| E. grandispicata H.P.Linder | - |
| E. hookeriana (Mast.) Moline and H.P.Linder | Chondropetalum hookerianum (Mast.) Pillans Dovea bolusii Mast. D. hookeriana Mast. |
| E. hutchinsonii Pillans | - |
| E. intermedia (Steud.) Pillans | E. membranacea (Nees) Kunth. E. membranaceus Nees Restio intermedius Steud. R. membranaceus Nees |
| E. juncea L. | E. junacea L. E. propinqua (Nees) Kunth Elegia juncea L. var. geniculate Pillans Elegia propinqua (Nees) Kunth var. minor Mast. Restio elegia Murray Restio junceus (L.) Nees R. propinquus Nees |
| E. macrocarpa (Kunth) Moline and H.P.Linder | Chondropetalum macrocarpum (Kunth) Kunth Dovea macrocarpa Kunth |
| E. marlothii (Pillans) Moline and H.P.Linder | Chondropetalum marlothii (Pillans) Pillans Dovea marlothii Pillans |
| E. microcarpa (Kunth) Moline and H.P.Linder | Chondropetalum microcarpum (Kunth) Pillans Dovea rigens Mast. D. microcarpa Kunth |
| E. mucronata (Nees) Kunth | Chondropetalum mucronatum (Nees) Pillans Dovea mucronata (Nees) Mast. E. panicoides Kunth Restio mucronatus Nees |
| E. muirii Pillans | - |
| E. namaquense H.P.Linder and Helme | - |
| E. neesii Mast. | Lamprocaulis neesii (Mast.) Mast. L. schlechteri Gilg-Ben. |
| E. nuda (Rottb.) Kunth | Chondropetalum nudum Rottb. Cuculifera dura Nees Dovea nuda (Rottb.) Pillans E. elongata Mast. Restio acuminatus Thunb. R. nudus (Rottb.) Nees |
| E. persistens Mast. | - |
| E. prominens Pillans | - |
| E. racemosa (Poir.) Pers. | Dovea racemosa (Poir.) Mast. E. bella Pillans E. fusca N.E. Br. E. racemosa (Poir.) Pers. Restio racemosa Poir. |
| E. recta (Mast.) Moline and H.P.Linder | Chondropetalum rectum (Mast.) Pillans Dovea recta Mast. |
| E. rigida Mast. | E. obtusiflora Mast. E. parviflora Pillans var. rigida (Mast.) E. spathacea Mast. var. attenuata Pillans |
| E. spathacea Mast. | - |
| E. squamosa Mast. | E. pectinata Pillans |
| E. stipularis Mast. | - |
| E. stokoei Pillans | - |
| E. tectorum (L.f.) Moline and H.P.Linder | Chondropetalum tectorum (L.f) Raf. Dovea cylindrostachya Mast. D. tectorum (L.f.) Mast. E. tectorumi (Mast.) Moline and H.P. Linder Restio tectorum L.f. |
| E. thyrsifera (Rotth.) Pers. | E. acuminata Mast. Restio thyrsifer Rottb. |
| E. thyrsoidea (Mast.) Pillans | Dovea thyrsoidea Mast. |
| E. vaginulata Mast. | - |
| E. verreauxii Mast. | - |
4. Traditional Uses
5. Phytochemistry
| Species | Compounds | Plant Part | References |
|---|---|---|---|
| E. capensis (Burm. F.) Schelpe | Myricetin 3-galactoside (5) Myricetin 3-arabinoside (6) Myricetin 3-rhamnoside (7) Quercetin 3-galactoside (4) Procyanidin * | stem, inflorescences | [11] |
| E. cuspidata Mast. | Syringetin 3-galactoside (12) Procyanidin * | stem, inflorescences | [11] |
| E. deustum (Rottb.) Kunth | Myricetin 3-galactoside (5) Myricetin 3-rhamnoside (6) Laricitrin 3-galactoside (9) Laricitrin 3-rhamnoside (10) Syringetin 3-galactoside (12) Syringetin 3-rhamnoside (14) Procyanidin * Prodelphinidin * | aerial culm # | [12] |
| E. filacea Mast. | Laricitrin 3-galactoside (9) Syringetin 3-galactoside (12) Procyanidin * Sulphated flavonoids * | stem, inflorescences | [11] |
| E. galpinii N.E.Br. | Myricetin 3-galactoside (5) Laricitrin 3-galactoside (9) Laricitrin 3-diglycoside * Syringetin 3-galactoside (12) Procyanidin * | stem, inflorescences | [11] |
| E. hookeriana (Mast.) Moline and H.P.Linder | Myricetin 3-galactoside (5) Myricetin 3-rhamnoside (7) Laricitrin 3-galactoside (9) Laricitrin 3-rhamnoside (10) Syringetin 3-galactoside (12) Syringetin 3-arabinoside (13) Cyanidin 3-glycoside (anthocyanin) * | stem, inflorescences; aerial culm # | [11,12] |
| E. macrocarpa (Kunth) Moline and H.P.Linder | Kaempferol (1) Quercetin (2) | aerial culm # | [12] |
| E. microcarpa (Kunth.) Moline and H.P.Linder | Myricetin 3-galactoside (5) Laricitrin 3-galactoside (9) Laricitrin 3-rhamnoside (10) Syringetin 3-galactoside (12) Syringetin 3-arabinoside (13) Procyanidin * Prodelphinidin * | aerial culm # | [12] |
| E. mucronata (Nees) Kunth | Myricetin 3-galactoside (5) Procyanidin * Prodelphinidin * | stem, inflorescences; aerial culm # | [11,12] |
| E. nuda (Rottb.) Kunth | Myricetin 3-galactoside (5) Myricetin 3-rhamnoside (7) Laricitrin 3-galactoside (9) Laricitrin 3-rhamnoside (10) Syringetin 3-galactoside (12) Procyanidin * Prodelphinidin * | aerial culm # | [12] |
| E. persistens Mast. | Myricetin 3-galactoside (5) Laricitrin 3-galactoside (9) Laricitrin 3-diglycoside * Syringetin 3-galactoside (12) Procyanidin * | stem, inflorescences | [11] |
| E. recta (Mast.) Moline and H.P.Linder | Myricetin 3-galactoside (5) Myricetin 3-rhamnoside (7) Laricitrin 3-galactoside (9) Laricitrin 3-rhamnoside (10) Syringetin 3-galactoside (12) Syringetin 3-arabinoside (13) Procyanidin * Prodelphinidin * | aerial culm # | [12] |
| E. spathacea Mast. | Syringetin 3-galactoside (12) | stem, inflorescences | [11] |
| E. tectorum (L.f.) Moline and H.P.Linder | Laricitrin 3-galactoside (9) Syringetin 3-galactoside (12) Procyanidin * Prodelphinidin * | stem, inflorescences; aerial culm # | [11,12] |
 | |||
|---|---|---|---|
| Chemical Name | R1 | R2 | R3 |
| Kaempferol (1) | OH | H | H |
| Quercetin (2) | OH | OH | H |
| Quercetin 3-galactoside (3) | O-gal | OH | H |
| Myricetin (4) | OH | OH | OH |
| Myricetin 3-galactoside (5) | O-gal | OH | OH |
| Myricetin 3-arabinoside (6) | O-ara | OH | OH |
| Myricetin 3-rhamnoside (7) | O-rha | OH | OH |
| Laricitrin (8) | OH | OMe | OH |
| Laricitrin 3-galactoside (9) | O-gal | OMe | OH |
| Laricitrin 3-rhamnoside (10) | O-rha | OMe | OH |
| Syringetin (11) | OH | OMe | OMe |
| Syringetin 3-galactoside (12) | O-gal | OMe | OMe |
| Syringetin 3-arabinoside (13) | O-ara | OMe | OMe |
| Syringetin 3-rhamnoside (14) | O-rha | OMe | OMe |
6. Potential Pharmacological Activities and Clinical Studies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dorrat-Haaksma, E.; Linder, H.P. Restios of the Fynbos, 2nd ed.; Struik Nature: Cape Town, South Africa, 2012. [Google Scholar]
- Moline, P.M.; Linder, H.P. Molecular Phylogeny and Generic Delimitation in the Elegia Group (Restionaceae, South Africa) Based on a Complete Taxon Sampling and Four Chloroplast DNA Regions. Syst. Bot. 2005, 30, 759–772. [Google Scholar] [CrossRef]
- Linder, H.P.; Briggs, B.G.; Johnson, L.A.S. The Families and Genera of Vascular Plants, 1st ed.; Kubitzki, K., Ed.; Springer: New York, NY, USA, 1998; Volume 4. [Google Scholar]
- Hardy, C.R.; Moline, P.; Linder, H.P. A Phylogeny for the African Restionaceae and New Perspectives on Morphology’s Role in Generating Complete Species Phylogenies for Large Clades. Int. J. Plant Sci. 2008, 169, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Linder, H.P.; Hardy, C.R. A Generic Classification of the Restioneae (Restionaceae), Southern Africa. Bothalia 2010, 40, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Briggs, B.; Linder, P. A New Subfamilial and Tribal Classification of Restionaceae (Poales). Telopea 2009, 12, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Linder, H.P. The Taxonomy of the African Restionaceae Available in Intkey Format. Available online: http://www.systbot.uzh.ch/en/Bestimmungsschluessel/Restionaceae (accessed on 13 October 2021).
- Steenkamp, V. Traditional Herbal Remedies Used by South African Women for Gynaecological Complaints. J. Ethnopharmacol. 2003, 86, 97–108. [Google Scholar] [CrossRef]
- Williams, V.L.; Victor, J.E.; Crouch, N.R. Red Listed Medicinal Plants of South Africa: Status, Trends, and Assessment Challenges. S. Afr. J. Bot. 2013, 86, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Aston Philander, L. An Ethnobotany of Western Cape Rasta Bush Medicine. J. Ethnopharmacol. 2011, 138, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Correlations between Flavonoid Chemistry, Anatomy and Geography in the Restionaceae. Phytochemistry 1979, 18, 1323–1327. [Google Scholar] [CrossRef]
- Harborne, J.B.; Boardley, M.; Linder, P. Variations in Flavonoid Patterns within the Genus Chondropetalum (Restionaceae). Phytochemistry 1985, 24, 6. [Google Scholar] [CrossRef]
- Harborne, J.B. Arsenal for Survival: Secondary Plant Products. Taxon 2000, 49, 435–449. [Google Scholar] [CrossRef]
- Harborne, J.B.; Clifford, H.T. Flavonoid Patterns of the Restionaceae. Gossypetin in Restio and a New Flavone in Hypolaena. Phytochemistry 1969, 8, 2071–2075. [Google Scholar] [CrossRef]
- Agrawal, A.D. Pharmacological Activities of Flavonoids: A Review. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1394–1398. [Google Scholar] [CrossRef]
- Brahmachari, G.; Gorai, D. Progress in the Research on Naturally Occurring Flavones and Flavonols: An Overview. COC 2006, 10, 873–898. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Tatsimo, S.J.N.; de Tamokou, J.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and Antioxidant Activity of Kaempferol Rhamnoside Derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [Green Version]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and Kaempferol Rhamnosides with Depigmenting and Anti-Inflammatory Properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semwal, D.; Semwal, R.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin Bioactive Effects: Moving from Preclinical Evidence to Potential Clinical Applications. BMC Complement. Med. 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Hayder, N.; Bouhlel, I.; Skandrani, I.; Kadri, M.; Steiman, R.; Guiraud, P.; Mariotte, A.-M.; Ghedira, K.; Dijoux-Franca, M.-G.; Chekir-Ghedira, L. In Vitro Antioxidant and Antigenotoxic Potentials of Myricetin-3-O-Galactoside and Myricetin-3-O-Rhamnoside from Myrtus communis: Modulation of Expression of Genes Involved in Cell Defence System Using CDNA Microarray. Toxicol. Vitr. 2008, 22, 567–581. [Google Scholar] [CrossRef]
- Xu, Z.; He, W.; Liu, C.; Kong, J. Enzymatic Synthesis of Myricetin 3-O-Galactoside through a Whole-Cell Biocatalyst. Chin. Herb. Med. 2020, 12, 384–389. [Google Scholar] [CrossRef]
- de Oliveira Azevedo, A.; Campos, J.J.; de Souza, G.G.; de Carvalho Veloso, C.; Duarte, I.D.G.; Braga, F.C.; de Castro Perez, A. Antinociceptive and Anti-Inflammatory Effects of Myricetin 3-O-β-Galactoside Isolated from Davilla elliptica: Involvement of the Nitrergic System. J. Nat. Med. 2015, 69, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Park, S.Y.; Seo, Y.; Kong, C.-S. Anticatabolic and Anti-Inflammatory Effects of Myricetin 3-O-β-D-Galactopyranoside in UVA-Irradiated Dermal Cells via Repression of MAPK/AP-1 and Activation of TGFβ/Smad. Molecules 2020, 25, 1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadeniz, F.; Oh, J.H.; Jo, H.J.; Seo, Y.; Kong, C.-S. Myricetin 3-O-β-D-Galactopyranoside Exhibits Potential Anti-Osteoporotic Properties in Human Bone Marrow-Derived Mesenchymal Stromal Cells via Stimulation of Osteoblastogenesis and Suppression of Adipogenesis. Cells 2021, 10, 2690. [Google Scholar] [CrossRef]
- Masuoka, C.; Yokoi, K.; Komatsu, H.; Kinjo, J.; Nohara, T.; Ono, M. Two Novel Antioxidant Ortho-Benzoyloxyphenyl Acetic Acid Derivatives from the Fruit of Vaccinium uliginosum. FSTR 2007, 13, 215–220. [Google Scholar] [CrossRef] [Green Version]
- do Vale, A.E.; David, J.M.; Brandão, H.N.; David, J.P. A New Flavonol Glycoside Derivative from Leaves of Moldenhawera Nutans. Z. Nat. C 2005, 60, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Lall, N. Natural Cosmetics from South African Wetland Plants; TT 817/20; Water Research Commission: Pretoria, South Africa, 2020. [Google Scholar]
| Keywords | Number of Searches/Hits | ||
|---|---|---|---|
| Google Scholar | PubMed | Reaxys | |
| Elegia | 88,300 | 7 | 38 |
| Restionaceae | 8550 | 50 | 238 |
| Restios | 1200 | 4 | 47 |
| Elegia compounds | 1180 | 1845 | 11 |
| Elegia phytochemicals | 32 | 61 | 0 |
| Elegia pharmacological | 191 | 5841 | 0 |
| Elegia traditional uses | 5750 | 30 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lymperis, P.; Tomou, E.-M.; De Canha, M.N.; Lall, N.; Skaltsa, H. Traditional Uses, Phytochemistry, and Pharmacology of Elegia Species: A Review. Sci. Pharm. 2022, 90, 4. https://doi.org/10.3390/scipharm90010004
Lymperis P, Tomou E-M, De Canha MN, Lall N, Skaltsa H. Traditional Uses, Phytochemistry, and Pharmacology of Elegia Species: A Review. Scientia Pharmaceutica. 2022; 90(1):4. https://doi.org/10.3390/scipharm90010004
Chicago/Turabian StyleLymperis, Panagiotis, Ekaterina-Michaela Tomou, Marco Nuno De Canha, Namrita Lall, and Helen Skaltsa. 2022. "Traditional Uses, Phytochemistry, and Pharmacology of Elegia Species: A Review" Scientia Pharmaceutica 90, no. 1: 4. https://doi.org/10.3390/scipharm90010004
APA StyleLymperis, P., Tomou, E. -M., De Canha, M. N., Lall, N., & Skaltsa, H. (2022). Traditional Uses, Phytochemistry, and Pharmacology of Elegia Species: A Review. Scientia Pharmaceutica, 90(1), 4. https://doi.org/10.3390/scipharm90010004