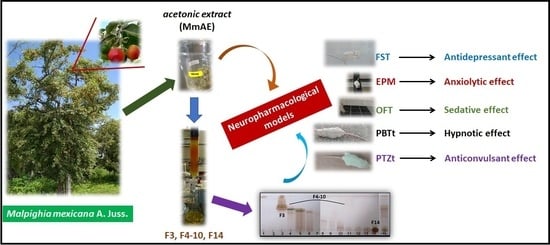
Neuropharmacological Activity of the Acetonic Extract of Malpighia mexicana A. Juss. and Its Phytochemical Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Identification
2.2. Preparation of MmAE Extract
2.3. MmAE Fractionation
2.4. HPLC and UPLC Analysis
2.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.6. Drugs and Chemicals
2.7. Animals
2.8. Neuropharmacological Assays
2.8.1. Forced Swimming Test (FST)
2.8.2. Elevated Plus Maze Test (EPM)
2.8.3. Open Field Test (OFT)
2.8.4. Pentobarbital-Induced Sleep Test (PBTt)
2.8.5. Pentylenetetrazol-Induced Seizure Test (PTZt)
2.9. Statistical Analysis
3. Results
3.1. HPLC Analysis
3.1.1. HPLC of MmAE
3.1.2. UPLC-MS Analysis
3.1.3. HPLC of Fraction 14
3.2. GC-MS of F3 and F4-10
3.3. Pharmacological Assays
3.3.1. FST
3.3.2. EPM
3.3.3. OFT
3.3.4. PBTt
3.3.5. PTZt
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bárcenas-López, L.Y.; Montaño-Arias, S.A.; López-Sandoval, J.A.; González, A.; Rubí-Arriaga, M.; Vargas, G. Anatomía foliar de Malpighia mexicana (Malpighiaceae). Acta Bot. Mex. 2019, 126, 1–12. [Google Scholar] [CrossRef]
- Maldonado-Peralta, M.Á.; García-de los Santos, G.; García-Nava, J.R.; Corona-Torres, T.; Cetina-Alcalá, V.M.; Ramírez-Herrera, C. Calidad morfológica de frutos y endocarpios del nanche rojo (Malpighia mexicana, Malpighiaceae). Acta Botánica Mex. 2016, 117, 37–46. [Google Scholar] [CrossRef]
- Dorado, O. Programa de Conservación y Manejo Reserva de la Biosfera Sierra de Huautla, 1st ed.; Comisión Natural de Áreas Naturales Protegidas: México City, México, 2005.
- Huerta-Reyes, M.; Zamilpa, A.; Álvarez-Chimal, R.; Luna-Manzanares, J.; León-Velasco, M.E.; Aguilar-Rojas, A.; Jiménez-Estrada, M.; Campos-Lara, M.G. Heteropterys cotinifolia: A neuropharmacological and phytochemical approach with possible taxonomic implications. Sci. World J. 2013, 2013, 870468. [Google Scholar] [CrossRef] [PubMed]
- BDMTM. Ajuaxocotl Malpighia mexicana Juss.—Malpighiaceae. Available online: http://www.medicinatradicionalmexicana.unam.mx/apmtm/termino.php?l=3&t=malpighia-mexicana (accessed on 17 March 2023).
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Ciências 2019, 91, 1–7. [Google Scholar] [CrossRef]
- Opio, J.N.; Munn, Z.; Aromataris, E. Prevalence of Mental Disorders in Uganda: A Systematic Review and Meta-Analysis. Psychiatr. Q. 2022, 93, 199–226. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Aeschbach, D. Sleep and anxiety: From mechanisms to interventions. Sleep Med. Rev. 2022, 61, 101583. [Google Scholar] [CrossRef]
- Bhatt, S.; Devadoss, T.; Manjula, S.N.; Rajangam, J. 5-HT(3) receptor antagonism a potential therapeutic approach for the treatment of depression and other disorders. Curr. Neuropharmacol. 2021, 19, 1545–1559. [Google Scholar] [CrossRef]
- Ziobrowski, H.N.; Kennedy, C.J.; Ustun, B.; House, S.L.; Beaudoin, F.L.; An, X.; Zeng, D.; Bollen, K.A.; Petukhova, M.; Sampson, N.A.; et al. Development and Validation of a Model to Predict Posttraumatic Stress Disorder and Major Depression After a Motor Vehicle Collision. JAMA Psychiatry 2021, 78, 1228–1237. [Google Scholar] [CrossRef]
- Choi, K.W.; Kim, Y.K.; Jeon, H.J. Comorbid Anxiety and Depression: Clinical and Conceptual Consideration and Transdiagnostic Treatment. Adv. Exp. Med. Biol. 2020, 1191, 219–235. [Google Scholar] [CrossRef]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Leichsenring, F.; Steinert, C.; Rabung, S.; Ioannidis, J.P.A. The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: An umbrella review and meta-analytic evaluation of recent meta-analyses. World Psychiatry 2022, 21, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Ijaz, S.; Williams, C.J.; Kessler, D.; Lewis, G.; Wiles, N. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst. Rev. 2019, 12, Cd010557. [Google Scholar] [CrossRef] [PubMed]
- Pham Nguyen, T.P.; Soprano, S.E.; Hennessy, S.; Brensinger, C.M.; Bilker, W.B.; Miano, T.A.; Acton, E.K.; Horn, J.R.; Chung, S.P.; Dublin, S.; et al. Population-based signals of benzodiazepine drug interactions associated with unintentional traumatic injury. J. Psychiatr. Res. 2022, 151, 299–303. [Google Scholar] [CrossRef]
- Friedman, J.H. Movement disorders induced by psychiatric drugs that do not block dopamine receptors. Park. Relat. Disord. 2020, 79, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Reyes, M.; Herrera-Ruiz, M.; González-Cortazar, M.; Zamilpa, A.; León, E.; Reyes-Chilpa, R.; Aguilar-Rojas, A.; Tortoriello, J. Neuropharmacological in vivo effects and phytochemical profile of the extract from the aerial parts of Heteropterys brachiata (L.) DC. (Malpighiaceae). J. Ethnopharmacol. 2013, 146, 311–317. [Google Scholar] [CrossRef]
- Gallegos-García, A.J.; Lobato-García, C.E.; González-Cortazar, M.; Herrera-Ruiz, M.; Zamilpa, A.; Álvarez-Fitz, P.; Pérez-García, M.D.; López-Rodríguez, R.; Ble-González, E.A.; Medrano-Sánchez, E.J.; et al. Preliminary Phytochemical Profile and Bioactivity of Inga jinicuil Schltdl & Cham. ex G. Don. Plants 2022, 11, 794. [Google Scholar] [CrossRef]
- Adams, R.P. (Ed.) Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Kallman, M.J. Despair Swim Test. In Drug Discovery and Evaluation: Pharmacological Assays, 4th ed.; Hock, F.J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1450–1453. [Google Scholar]
- Vogel, H.G.; Vogel, W.H. (Eds.) Antidepressant activity. In Drug Discovery and Evaluation: Pharmacological Assays; Springer: Berlin/Heidelberg, Germany, 1997; pp. 302–303. [Google Scholar]
- Kallman, M.J. Elevated Plus Maze Test. In Drug Discovery and Evaluation: Pharmacological Assays, 4th ed.; Hock, F.J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1127–1130. [Google Scholar]
- La-Vu, M.; Tobias, B.C.; Schuette, P.J.; Adhikari, A. To Approach or Avoid: An Introductory Overview of the Study of Anxiety Using Rodent Assays. Front. Behav. Neurosci. 2020, 14, 145. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Vogel, H.G.; Vogel, W.H. (Eds.) Open field test. In Drug Discovery and Evaluation: Pharmacological Assays; Springer: Berlin/Heidelberg, Germany, 1997; pp. 206–208. [Google Scholar]
- Kallman, M.J. Open Field Test. In Drug Discovery and Evaluation: Pharmacological Assays, 4th ed.; Hock, F.J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1044–1048. [Google Scholar]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef]
- Kallman, M.J. Potentiation of Hexobarbital Sleeping Time. In Drug Discovery and Evaluation: Pharmacological Assays, 4th ed.; Hock, F.J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1307–1308. [Google Scholar]
- Vogel, H.G.; Vogel, W.H. (Eds.) Potentiation of hexobarbital sleeping time. In Drug Discovery and Evaluation: Pharmacological Assays; Springer: Berlin/Heidelberg, Germany, 1997; pp. 267–268. [Google Scholar]
- Kallman, M.J. Anticonvulsant activity: Pentylenetetrazole metrazole-Induce Convulsions. In Drug Discovery and Evaluation: Pharmacological Assays, 4th ed.; Hock, F.J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1109–1111. [Google Scholar]
- Vogel, H.G.; Vogel, W.H. (Eds.) Anticonvulsant activity: Pentylentetrazole (Metrazole) induced convulsions. In Drug Discovery and Evaluation: Pharmacological Assays; Springer: Berlin/Heidelberg, Germany, 1997; pp. 227–228. [Google Scholar]
- Tortoriello, J.; Lozoya, X. Effect of Galphimia glauca methanolic extract on neuropharmacological tests. Planta Med. 1992, 58, 234–236. [Google Scholar] [CrossRef]
- Rios, M.Y.; Ortega, A.; Domínguez, B.; Déciga, M.; Rosa, V. Glaucacetalin E and galphimidin B from Galphimia glauca and their anxiolytic activity. J. Ethnopharmacol. 2020, 259, 112939. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Zamilpa, A.; González-Cortazar, M.; Reyes-Chilpa, R.; León, E.; García, M.P.; Tortoriello, J.; Huerta-Reyes, M. Antidepressant effect and pharmacological evaluation of standardized extract of flavonoids from Byrsonima crassifolia. Phytomedicine 2011, 18, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Galietta, G.; Giuliani, G.; Loizzo, A.; Amat, A.G.; Fumagalli, E.; De Feo, V.; Quaranta, E.; Paladino, L.; Capasso, A. Neurophysiological studies of Heteropteris glabra Hok. & Arn. (Malpighiaceae) in DBA/2J mice. J. Ethnopharmacol. 2005, 97, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ruiz, M.; Jiménez-Ferrer, J.E.; De Lima, T.C.; Avilés-Montes, D.; Pérez-García, D.; González-Cortazar, M.; Tortoriello, J. Anxiolytic and antidepressant-like activity of a standardized extract from Galphimia glauca. Phytomedicine 2006, 13, 23–28. [Google Scholar] [CrossRef]
- Avilés-Montes, D.; Herrera-Ruiz, M.; Román-Ramos, R.; Jiménez-Ferrer, E.; González-Cortazar, M.; Zamilpa, A.; Tortoriello, J. Pharmacological Interaction between Galphimine-A, a Natural AnxiolyticCompound and Gabaergic Drugs. Int. J. Pharmacol. 2015, 11, 944–955. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R. Acerola, an untapped functional superfruit: A review on latest frontiers. J. Food Sci. Technol. 2018, 55, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Santos-Buelga, C.; González-Paramás, A.M.; Forbes-Hernández, T.Y.; Afrin, S.; Páez-Watson, T.; Quiles, J.L.; et al. The protective effect of acerola (Malpighia emarginata) against oxidative damage in human dermal fibroblasts through the improvement of antioxidant enzyme activity and mitochondrial functionality. Food Funct. 2017, 8, 3250–3258. [Google Scholar] [CrossRef]
- Liu, J.Q.; Peng, X.R.; Li, X.Y.; Li, T.Z.; Zhang, W.M.; Shi, L.; Han, J.; Qiu, M.H. Norfriedelins A-C with acetylcholinesterase inhibitory activity from acerola tree (Malpighia emarginata). Org. Lett. 2013, 15, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Costa, J.P.; Ferreira, P.B.; De Sousa, D.P.; Jordan, J.; Freitas, R.M. Anticonvulsant effect of phytol in a pilocarpine model in mice. Neurosci. Lett. 2012, 523, 115–118. [Google Scholar] [CrossRef]
- Violi, F.; Nocella, C.; Loffredo, L.; Carnevale, R.; Pignatelli, P. Interventional study with vitamin E in cardiovascular disease and meta-analysis. Free Radic. Biol. Med. 2022, 178, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Nath, N.; Rauf, A.; Emran, T.B.; Mitra, S.; Islam, F.; Chandran, D.; Barua, J.; Khandaker, M.U.; Idris, A.M.; et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem. Biol. Interact. 2022, 365, 110117. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-Safi, I.; Boukhira, S.; Aliqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Conte, R.; Calarco, A.; Bousta, D. Subacute Assessment of the Toxicity and Antidepressant-Like Effects of Origanum Majorana L. Polyphenols in Swiss Albino Mice. Molecules 2020, 25, 5653. [Google Scholar] [CrossRef] [PubMed]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Devi, S.; Salkini, M.A.; Alam, P. Rutin Improves Anxiety and Reserpine-Induced Depression in Rats. Molecules 2022, 27, 7313. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, J.; Liang, L.F.; Shen, S.Y.; Li, W.; Chen, X.R.; Li, B.; Zhang, Y.Q.; Yu, J. Sirtuin 3 Plays a Critical Role in the Antidepressant- and Anxiolytic-like Effects of Kaempferol. Antioxidants 2022, 11, 1886. [Google Scholar] [CrossRef] [PubMed]
- Pereira Costa, J.; da Silva Oliveira, J.; Mario Rezende Junior, L.; de Freitas, R.M. Phytol a Natural Diterpenoid with Pharmacological Applications on Central Nervous System: A Review. Recent. Pat. Biotechnol. 2014, 8, 194–205. [Google Scholar] [CrossRef]
- Pérez-Davison, G.; Restrepo-Manrique, R.; Martínez-Sánchez, G. Hormesis: Antecedentes e Implicaciones en los Sistemas Biológicos. Lat. Am. J. Pharm. 2009, 28, 954–960. [Google Scholar]
- Chirumbolo, S. Hormesis, resveratrol and plant-derived polyphenols: Some comments. Hum. Exp. Toxicol. 2011, 30, 2027–2030. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Dose response biology: The case of resveratrol. Hum. Exp. Toxicol. 2010, 29, 1034–1037. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Islas-Garduño, A.L.; Zamilpa, A.; Pérez-García, M.D.; Tortoriello, J. Therapeutic Effectiveness of Galphimia glauca in Young People with Social Anxiety Disorder: A Pilot Study. Evid. Based Complement. Altern. Med. 2018, 2018, 1716939. [Google Scholar] [CrossRef] [PubMed]
- Ortíz, A.; Cardoso-Taketa, A.; Monroy, M.R.; Arellano, J.; Hernández, G.; Villarreal, M.L. Transformed cell suspension culture of Galphimia glauca producing sedative nor-friedelanes. Planta Med. 2010, 76, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Noguerón-Merino, M.C.; Jiménez-Ferrer, E.; Román-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Herrera-Ruiz, M. Interactions of a standardized flavonoid fraction from Tilia americana with Serotoninergic drugs in elevated plus maze. J. Ethnopharmacol. 2015, 164, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Shri, R.; Kamboj, A. Bioactivity-directed isolation, characterization, and quantification of an anxiolytic flavonoid from Brassica oleracea L. J. Food Biochem. 2021, 45, e13608. [Google Scholar] [CrossRef]
- Hernandez-Leon, A.; González-Trujano, M.E.; Fernández-Guasti, A. The anxiolytic-like effect of rutin in rats involves GABAA receptors in the basolateral amygdala. Behav. Pharmacol. 2017, 28, 303–312. [Google Scholar] [CrossRef]
- Pérez-Ortega, G.; Angeles-López, G.E.; Argueta-Villamar, A.; González-Trujano, M.E. Preclinical evidence of the anxiolytic and sedative-like activities of Tagetes erecta L. reinforces its ethnobotanical approach. Biomed. Pharmacother. 2017, 93, 383–390. [Google Scholar] [CrossRef]
- Islam, M.S.; Hossain, R.; Ahmed, T.; Rahaman, M.M.; Al-Khafaji, K.; Khan, R.A.; Sarkar, C.; Bappi, M.H.; de Andrade, E.M.; Araújo, I.M.; et al. Anxiolytic-like Effect of Quercetin Possibly through GABA Receptor Interaction Pathway: In Vivo and In Silico Studies. Molecules 2022, 27, 7149. [Google Scholar] [CrossRef]
- Grundmann, O.; Nakajima, J.; Kamata, K.; Seo, S.; Butterweck, V. Kaempferol from the leaves of Apocynum venetum possesses anxiolytic activities in the elevated plus maze test in mice. Phytomedicine 2009, 16, 295–302. [Google Scholar] [CrossRef]
- Costa, J.P.; de Oliveira, G.A.L.; de Almeida, A.A.C.; Islam, M.T.; de Sousa, D.P.; de Freitas, R.M. Anxiolytic-like effects of phytol: Possible involvement of GABAergic transmission. Brain Res. 2014, 1547, 34–42. [Google Scholar] [CrossRef]
- Tatem, K.S.; Quinn, J.L.; Phadke, A.; Yu, Q.; Gordish-Dressman, H.; Nagaraju, K. Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. J. Vis. Exp. 2014, e51785. [Google Scholar] [CrossRef]
- Chaves, E.M.C.; Honório-Júnior, J.E.R.; Sousa, C.N.S.; Monteiro, V.S.; Nonato, D.T.T.; Dantas, L.P.; Lúcio, A.; Barbosa-Filho, J.M.; Patrocínio, M.C.A.; Viana, G.S.B.; et al. The anxiolytic-like effect of 6-styryl-2-pyrone in mice involves GABAergic mechanism of action. Metab. Brain Dis. 2018, 33, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Riaz, T.A.; Ayatollahi, S.A.; Sharifi-Rad, J. Anxiolytic-like effect of Urena lobata (L.) in Swiss albino mice. Clin. Phytoscience 2021, 7, 11. [Google Scholar] [CrossRef]
- Islam, M.T.; Martins, N.; Imran, M.; Hameed, A.; Ali, S.W.; Salehi, B.; Ahmad, I.; Hussain, A.; Sharifi-Rad, J. Anxiolytic-like effects of Moringa oleifera in Swiss mice. Cell Mol Biol 2020, 66, 73–77. [Google Scholar] [CrossRef]
- Jeon, S.J.; Park, H.J.; Gao, Q.; Lee, H.E.; Park, S.J.; Hong, E.; Jang, D.S.; Shin, C.Y.; Cheong, J.H.; Ryu, J.H. Positive effects of β-amyrin on pentobarbital-induced sleep in mice via GABAergic neurotransmitter system. Behav. Brain Res. 2015, 291, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Wu, B.F.; Shang, J.H.; Zhao, Y.L.; Huang, A.X. Moringa oleifera Lam Seed Oil Augments Pentobarbital-Induced Sleeping Behaviors in Mice via GABAergic Systems. J. Agric. Food Chem. 2020, 68, 3149–3162. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.E.; Mabunga, D.F.; Kim, H.J.; Han, S.H.; Kim, H.Y.; Shin, C.Y.; Kwon, K.J. Novel Therapeutics for Treating Sleep Disorders: New Perspectives on Maydis stigma. Int. J. Mol. Sci. 2022, 23, 4612. [Google Scholar] [CrossRef]
- Akyuz, E.; Paudel, Y.N.; Polat, A.K.; Dundar, H.E.; Angelopoulou, E. Enlightening the neuroprotective effect of quercetin in epilepsy: From mechanism to therapeutic opportunities. Epilepsy Behav. 2021, 115, 107701. [Google Scholar] [CrossRef]
- Cárdenas-Rodríguez, N.; González-Trujano, M.E.; Aguirre-Hernández, E.; Ruíz-García, M.; Sampieri, A., 3rd; Coballase-Urrutia, E.; Carmona-Aparicio, L. Anticonvulsant and antioxidant effects of Tilia americana var. mexicana and flavonoids constituents in the pentylenetetrazole-induced seizures. Oxid. Med. Cell Longev. 2014, 2014, 329172. [Google Scholar] [CrossRef]
- Nieoczym, D.; Socała, K.; Raszewski, G.; Wlaź, P. Effect of quercetin and rutin in some acute seizure models in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 50–58. [Google Scholar] [CrossRef]
- Sumbul, O.; Aygun, H. Chronic effects of different quercetin doses in penicillin-induced focal seizure model. Neurosci. Lett. 2021, 753, 135848. [Google Scholar] [CrossRef]
- Tavakoli, Z.; Tahmasebi Dehkordi, H.; Lorigooini, Z.; Rahimi-Madiseh, M.; Korani, M.S.; Amini-Khoei, H. Anticonvulsant effect of quercetin in pentylenetetrazole (PTZ)-induced seizures in male mice: The role of anti-neuroinflammatory and anti-oxidative stress. Int. Immunopharmacol. 2023, 116, 109772. [Google Scholar] [CrossRef] [PubMed]
- Bang, M.H.; Choi, S.Y.; Jang, T.O.; Kim, S.K.; Kwon, O.S.; Kang, T.C.; Won, M.H.; Park, J.; Baek, N.I. Phytol, SSADH inhibitory diterpenoid of Lactuca sativa. Arch. Pharm. Res. 2002, 25, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Aragão, G.F.; Carneiro, L.M.; Rota-Junior, A.P.; Bandeira, P.N.; de Lemos, T.L.; Viana, G.S. Alterations in brain amino acid metabolism and inhibitory effects on PKC are possibly correlated with anticonvulsant effects of the isomeric mixture of α- and β-amyrin from Protium heptaphyllum. Pharm. Biol. 2015, 53, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.d.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.C.; de Oliveira, G.A.L.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.P.; Costa, D.A.; Almeida, R.N.; Freitas, R.M.; Sousa, D.P.; Fortes, A.C.; Patrício, C.C.M.; Soares, M.F.L.R. Applications of Phytol in Pharmaceutical Formulations Anxiolytic and Antidepressant Properties. Chem. Biol. Interact. 2011, 240, 63–70. [Google Scholar]
- Park, S.J.; Ahn, Y.J.; Oh, S.R.; Lee, Y.; Kwon, G.; Woo, H.; Lee, H.E.; Jang, D.S.; Jung, J.W.; Ryu, J.H. Amyrin attenuates scopolamine-induced cognitive impairment in mice. Biol. Pharm. Bull. 2014, 37, 1207–1213. [Google Scholar] [CrossRef]
- Simão da Silva, K.A.B.; Paszcuk, A.F.; Passos, G.F.; Silva, E.S.; Bento, A.F.; Meotti, F.C.; Calixto, J.B. Activation of cannabinoid receptors by the pentacyclic triterpene α,β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. Pain 2011, 152, 1872–1887. [Google Scholar] [CrossRef]
- Park, H.J.; Kwon, H.; Lee, J.H.; Cho, E.; Lee, Y.C.; Moon, M.; Jun, M.; Kim, D.H.; Jung, J.W. β-Amyrin Ameliorates Alzheimer’s Disease-Like Aberrant Synaptic Plasticity in the Mouse Hippocampus. Biomol. Ther. 2020, 28, 74–82. [Google Scholar] [CrossRef]
- Lee, A.; Tariq, A.; Lau, G.; Tok, N.W.K.; Tam, W.W.S.; Ho, C.S.H. Vitamin E, Alpha-Tocopherol, and Its Effects on Depression and Anxiety: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 656. [Google Scholar] [CrossRef]
- Vanmierlo, T.; Weingärtner, O.; van der Pol, S.; Husche, C.; Kerksiek, A.; Friedrichs, S.; Sijbrands, E.; Steinbusch, H.; Grimm, M.; Hartmann, T.; et al. Dietary intake of plant sterols stably increases plant sterol levels in the murine brain. J. Lipid Res. 2012, 53, 726–735. [Google Scholar] [CrossRef]
- Sharma, N.; Tan, M.A.; An, S.S.A. Phytosterols: Potential Metabolic Modulators in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 12255. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Wang, Y. Sterolomics in biology, biochemistry, medicine. Trends Anal. Chem. 2019, 120, 115280. [Google Scholar] [CrossRef] [PubMed]
- Panayotis, N.; Freund, P.A.; Marvaldi, L.; Shalit, T.; Brandis, A.; Mehlman, T.; Tsoory, M.M.; Fainzilber, M. β-sitosterol reduces anxiety and synergizes with established anxiolytic drugs in mice. Cell Rep. Med. 2021, 2, 100281. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Peralta, M.A.; De Los Santos, G.G.; García-Nava, J.R.; Ramírez-Herrera, C.; Hernández-Livera, A.; Valdez-Carrazco, J.M.; Corona-Torres, T.; Cetina-Alcalá, V.M. Seed viability and vigour of two nanche species (Malpighia mexicana and Byrsonima crassifolia). Seed Sci. Technol. 2016, 44, 168–176. [Google Scholar] [CrossRef]
- Maldonado-Peralta, M.A.; García De Los Santos, G.; García-Nava, J.R.; Rojas-García, A.R. Propagación vegetativa de nanche Malpighia mexicana y Byrsonima crassifolia. Rev. Mex. Cienc. Agrícolas 2017, 8, 611–619. [Google Scholar] [CrossRef]















| Peak | Compound | Retention Time (min) | Relative Area (%) | Correlation (%) | Mass (g/mol) | Chemical Structure |
|---|---|---|---|---|---|---|
| 1 | 6,10,14-trimethylpentadecan-2-one | 17.692 | 0.5 | 2.0 | 268 |  |
| 2 | phytol | 20.346 | 13.9 | 52.6 | 296 |  |
| 3 | 4,8,12,16- tetramethylheptadecan-4-olide | 22.467 | 0.7 | 3.0 | 324 |  |
| 4 | squalene oxide | 30.304 | 2.2 | 8.4 | 426 |  |
| 5 | β-tocopherol | 31.591 | 1.6 | 6.2 | 416 |  |
| 6 | α-tocopherol | 32.747 | 26.5 | 100 | 430 |  |
| 7 | β-sitosterol | 35.664 | 2.7 | 10.3 | 414 |  |
| 8 | β-amyrin | 36.301 | 12.3 | 46.6 | 426 |  |
| 9 | α-amyrin | 37.148 | 3.6 | 13.7 | 426 |  |
| Peak | Compound | Retention Time (min) | Relative Area (%) | Correlation (%) | Mass (g/mol) | Chemical Structure |
|---|---|---|---|---|---|---|
| 1 | phytol | 20.313 | 10.7 | 39.4 | 296 |  |
| 2 | 4,8,12,16-tetramethylheptadecan-4-olide | 22.467 | 1.2 | 4.7 | 324 |  |
| 3 | α-tocopherol | 32.635 | 27.2 | 100 | 430 |  |
| 4 | β-sitosterol | 35.591 | 9.6 | 35.2 | 414 |  |
| 5 | β-amyrin | 36.169 | 2.9 | 10.4 | 426 |  |
| Treatment (mg/kg) | Latency of Convulsions (s) | Mortality Protection (%) |
|---|---|---|
| Veh (100 µL/10 g) | 54.00 ± 6.22 a | 0.00 |
| DZP (1.0) | 131.75 ± 7.68 b | 100.00 |
| MmAE (50) | 55.00 ± 2.83 a | 50.00 |
| MmAE (100) | 54.50 ± 3.39 a | 75.00 |
| MmAE (150) | 49.60 ± 5.55 a | 37.50 |
| MmAE (200) | 53.20 ± 5.45 a | 87.50 |
| MmAE (250) | 52.50 ± 6.45 a | 50.00 |
| Treatment (mg/kg) | Latency of Convulsions (s) | Mortality Protection (%) |
|---|---|---|
| DZP (1.0) | 131.75 ± 7.68 b | 100.00 |
| Veh (100 µL/10 g) | 55.00 ± 05.45 a | 0 |
| F3 (100) | 64.00 ± 10.84 a | 100.00 |
| F4-10 (100) | 66.16 ± 03.25 a | 83.33 |
| F14 (100) | 71.66 ± 11.46 a | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avilés-Montes, D.; Salinas-Sánchez, D.O.; Sotelo-Leyva, C.; Zamilpa, A.; Batalla-Martinez, F.I.; Abarca-Vargas, R.; Rivas-González, J.M.; Dorado, Ó.; Figueroa-Brito, R.; Petricevich, V.L.; et al. Neuropharmacological Activity of the Acetonic Extract of Malpighia mexicana A. Juss. and Its Phytochemical Profile. Sci. Pharm. 2023, 91, 47. https://doi.org/10.3390/scipharm91040047
Avilés-Montes D, Salinas-Sánchez DO, Sotelo-Leyva C, Zamilpa A, Batalla-Martinez FI, Abarca-Vargas R, Rivas-González JM, Dorado Ó, Figueroa-Brito R, Petricevich VL, et al. Neuropharmacological Activity of the Acetonic Extract of Malpighia mexicana A. Juss. and Its Phytochemical Profile. Scientia Pharmaceutica. 2023; 91(4):47. https://doi.org/10.3390/scipharm91040047
Chicago/Turabian StyleAvilés-Montes, Dante, David Osvaldo Salinas-Sánchez, César Sotelo-Leyva, Alejandro Zamilpa, Franceli Itzel Batalla-Martinez, Rodolfo Abarca-Vargas, Juan Manuel Rivas-González, Óscar Dorado, Rodolfo Figueroa-Brito, Vera L. Petricevich, and et al. 2023. "Neuropharmacological Activity of the Acetonic Extract of Malpighia mexicana A. Juss. and Its Phytochemical Profile" Scientia Pharmaceutica 91, no. 4: 47. https://doi.org/10.3390/scipharm91040047
APA StyleAvilés-Montes, D., Salinas-Sánchez, D. O., Sotelo-Leyva, C., Zamilpa, A., Batalla-Martinez, F. I., Abarca-Vargas, R., Rivas-González, J. M., Dorado, Ó., Figueroa-Brito, R., Petricevich, V. L., Morales-Ferra, D. L., & González-Cortazar, M. (2023). Neuropharmacological Activity of the Acetonic Extract of Malpighia mexicana A. Juss. and Its Phytochemical Profile. Scientia Pharmaceutica, 91(4), 47. https://doi.org/10.3390/scipharm91040047